��Ŀ����
��þ�����Ļ����0.1 mol����100 mL 2 mol/L��H2S04��Һ�У�Ȼ���ٵμ�1 mol/L��NaOH��Һ����ش�
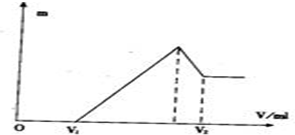
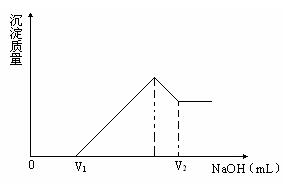
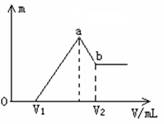
��1�����ڵμ�NaOH��Һ�Ĺ����У��������������NaOH��Һ�����V�仯����ͼ��ʾ����V1=160 mLʱ���������ĩ�У�n(Mg) =�� ��mol��V2=�� ��mL��
��2�����ڵμ�NaOH��Һ�Ĺ����У���ʹMg2+��A13 +�պó�����ȫ�������NaOH��Һ�����Ϊ����
��1��0.06 440 ��2��400
����:

��ϰ��ϵ�д�
�����Ŀ