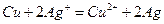��Ŀ����
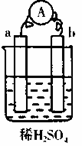
��ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺
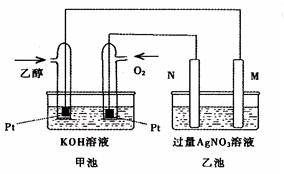
��1��M�缫�IJ����� ���缫������ ��N�ĵ缫��ӦʽΪ �������Ҵ��IJ��缫�ĵ缫��Ӧʽ ��
��2���ڴ˹����У��ҳ���ijһ�缫����������4.32gʱ���׳�����������������Ϊ L����״���£�������ʱ�ҳ���Һ�����Ϊ400mL�����ҳ�����Һ��pHΪ ��
��3�����ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29.71kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��

��1��M�缫�IJ����� ���缫������ ��N�ĵ缫��ӦʽΪ �������Ҵ��IJ��缫�ĵ缫��Ӧʽ ��
��2���ڴ˹����У��ҳ���ijһ�缫����������4.32gʱ���׳�����������������Ϊ L����״���£�������ʱ�ҳ���Һ�����Ϊ400mL�����ҳ�����Һ��pHΪ ��
��3�����ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29.71kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��1���� ���� 4OH����4e�� 2H2O+O2��
C2H5OH��12e+16OH��=2CO32��+11H2O��2�֣�
��2��0.224 1
��3��C2H5OH��l��+3O2��g�� 2CO2��g��+3H2O��l����H=��1366.7kJ/mol
C2H5OH��12e+16OH��=2CO32��+11H2O��2�֣�
��2��0.224 1
��3��C2H5OH��l��+3O2��g�� 2CO2��g��+3H2O��l����H=��1366.7kJ/mol
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ