��Ŀ����
PMMA�׳��л�������������Ϊֹ�ϳ����������ʵ��������һ�֣���ϳ���·����ͼ��ʾ��
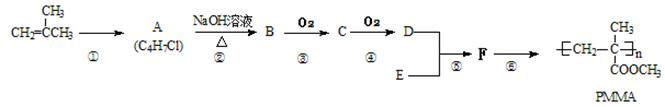
��֪����һ�������¿��Է������·�Ӧ��
(X����±��)
�ش��������⣺
��1�� F�Ľṹ��ʽΪ �����������ŵ������� ��
��2��д��A�Ľṹ��ʽ ���ܵķ�Ӧ���� ��
��3��д���ۡ��ݷ�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
��4���л���N��ʹ��ˮ�����Ը��������ɫ�������л���M��Ϊͬ���칹�壬��д������Ҫ�������N�Ľṹ��ʽ ��

��֪����һ�������¿��Է������·�Ӧ��

(X����±��)
�ش��������⣺
��1�� F�Ľṹ��ʽΪ �����������ŵ������� ��
��2��д��A�Ľṹ��ʽ ���ܵķ�Ӧ���� ��
��3��д���ۡ��ݷ�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
��4���л���N��ʹ��ˮ�����Ը��������ɫ�������л���M��Ϊͬ���칹�壬��д������Ҫ�������N�Ľṹ��ʽ ��
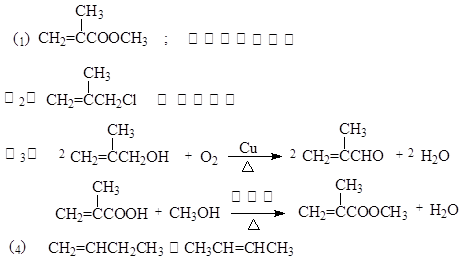
����������������ƺ��������ϵķ���������л�������ʺͷ�Ӧ�������Ʋ�������л��Ȼ������л���Ľṹ�����ʴ��⡣ת����ϵΪ��
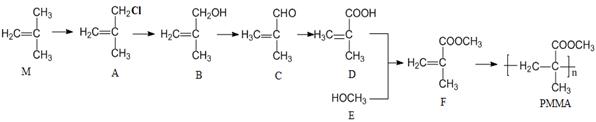

��ϰ��ϵ�д�
�����Ŀ
 ��C��C��CH��CH��CH3���й���ṹ˵����ȷ����
��C��C��CH��CH��CH3���й���ṹ˵����ȷ����
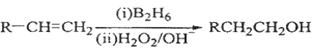

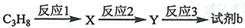
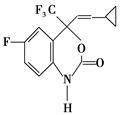
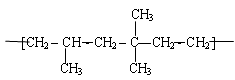 �����䵥�岻����Ϊ�� ��
�����䵥�岻����Ϊ�� ��

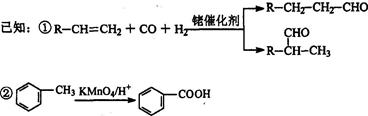
 ����
�м���� 2E+4CO2+4H2O��������X��һ�����ֻ�����֡�X�ķ���ʽ��___________��
2E+4CO2+4H2O��������X��һ�����ֻ�����֡�X�ķ���ʽ��___________��