��Ŀ����
����Ŀ��������Һ�и�����Ũ�ȹ�ϵ��ȷ����(����)
A. ��pH�İ�ˮ��KOH��Һ��Ba(OH)2��Һ�У�c(NH)��c(K��)>c(Ba2��)
B. ��10 mL 0.1 mol��L��1Na2CO3��Һ��εμӵ�10 mL 0.1 mol��L��1�����У�c(Na��)>c(Cl��)>c(HCO)>c(CO![]() )
)
C. ��NH4HCO3��Һ�еμ�NaOH��Һ��pH��7��c(NH)��c(Na��)��c(HCO)��c(CO![]() )
)
D. 0.2 mol��L��1��ijһԪ����HA��Һ��0.1 mol��L��1NaOH��Һ�������Ϻ����Һ��2c(OH��)��c(A��)��2c(H��)��c(HA)
���𰸡�AD
��������A. ��pH�İ�ˮ��KOH��Һ��Ba(OH)2��Һ��c(Cl��)�����c(NH)��c(K��)>c(Ba2��)����A��ȷ��B. ��10 mL 0.1 mol��L��1Na2CO3��Һ��εμӵ�10 mL 0.1 mol��L��1�����У�������Ӧ��Na2CO3+2HCl![]() 2NaCl+H2O+CO2����ʣ��Na2CO3��Na2CO3����ˮ����c(Na��)>c(Cl��) >c(CO
2NaCl+H2O+CO2����ʣ��Na2CO3��Na2CO3����ˮ����c(Na��)>c(Cl��) >c(CO![]() )>c(HCO)����B����C. ��NH4HCO3��Һ�еμ�NaOH��Һ��pH��7��c(H��)��c(OH��)������غ㣺c(NH)��c(Na��)��c(H��)��c(HCO)��2c(CO
)>c(HCO)����B����C. ��NH4HCO3��Һ�еμ�NaOH��Һ��pH��7��c(H��)��c(OH��)������غ㣺c(NH)��c(Na��)��c(H��)��c(HCO)��2c(CO![]() )��c(OH��)������c(NH)��c(Na��)��c(HCO)��c(CO
)��c(OH��)������c(NH)��c(Na��)��c(HCO)��c(CO![]() )����C����D. 0.2 mol��L��1��ijһԪ����HA��Һ��0.1 mol��L��1NaOH��Һ�������Ϻ��γɵ�Ũ�ȵ�HA��NaA�Ļ����Һ������غ㣺c(OH��)��c(A��)��c(H��)��c(Na+)��Ԫ���غ㣺c(Na+)=c(A��)��c(HA)����ȥc(Na+)�ɵ�2c(OH��)��c(A��)��2c(H��)��c(HA)����D��ȷ����ѡAD��
)����C����D. 0.2 mol��L��1��ijһԪ����HA��Һ��0.1 mol��L��1NaOH��Һ�������Ϻ��γɵ�Ũ�ȵ�HA��NaA�Ļ����Һ������غ㣺c(OH��)��c(A��)��c(H��)��c(Na+)��Ԫ���غ㣺c(Na+)=c(A��)��c(HA)����ȥc(Na+)�ɵ�2c(OH��)��c(A��)��2c(H��)��c(HA)����D��ȷ����ѡAD��

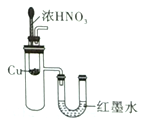
����Ŀ������ʵ��װ�ò��ܴﵽʵ��Ŀ�ĵ���
A | B | C | D |
|
|
|
|
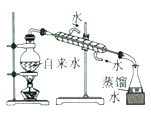
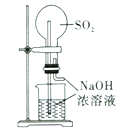
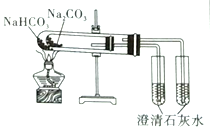
ʵ������ȡ����ˮ | ��SO2��NaOH��Һ����Ȫʵ�� | ֤��Na2CO3�����ȶ��Ա�NaHCO3�� | ֤��ͭ��Ũ����ķ�Ӧ�Ƿ��ȷ�Ӧ |
A. A B. B C. C D. D