��Ŀ����
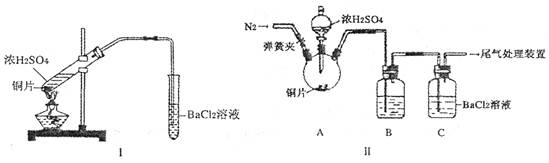
(12��) ijһ��Ӧ��ϵ�У��з�Ӧ��������ﹲ�������ʣ������������ǣ�Cl2��KMnO4��MnCl2��H2O��HCl(Ũ)��KCl������KMnO4 �� MnCl2 ��
(1)�÷�Ӧ�еĻ�ѧ����ʽΪ_____________________________________________��
(2)�÷�Ӧ�У����������뻹ԭ��������ʵ���֮��Ϊ_____________________________��
(3)�������������ڱ�״�������Ϊ2.24 L����Ӧ�����б�����������Ϊ________ NA��NA��ʾ����٤��������ֵ����
(4)���۵�Ũ���ᣨ�ܶ�Ϊ1.19g/cm3���ڹ�ҵ������500 L HCl����(��״��)��1 L H2O�ı������Ƴɵģ������������ʵ���Ũ����___________mol/L(�������һλС��)��
(5)������1.20 mol/L��ϡ����480 mL, Ӧ��ȡ����Ũ����________mL�������ơ�
(5)ȡ6.00 gӲ���Ͻ𣨼���ֻ����ͭ�裩���� (5) ������ϡ������г�ַ�Ӧ���ռ�������5.60 L(��״��)����Ӳ���Ͻ���������������Ϊ____________��
(��12��) (1) 2KMnO4 + 16HCl = 2KCl + 2MnCl2 + 5Cl2�� +8H2O��2�֣�
(2) 5��2��2�֣�(3) 0.2��2�֣�(4) 14.6��2�֣�
(5) 41.1��2�֣�(6) 75%��2�֣�
��������

 mL, Ӧ��ȡ����Ũ����________mL�������ơ�
mL, Ӧ��ȡ����Ũ����________mL�������ơ�
 ��������������
�������������� ��
��