��Ŀ����
ʵ������Ҫ480mL0.1mol?L-1������ͭ��Һ����ѡȡ500mL����ƿ�������ƣ�
��1����Ҫ����������Ϊ
��2���ڸ�ʵ����Ҫ�õ�����ƿ��ʹ������ƿ�ĵ�һ����
��3����Դ����ƹ��������5���������裺����ʢ�е������ձ��м�����ˮʹ���ܽ⣻�ڼ���������ƿ�м�ˮ��Һ��ӽ��̶���1-2cm���۽�����ͭ��Һ�ز�����ע��500mL����ƿ�У������ձ��м�������ˮ��С��ϴ��2-3�κ���������ƿ�У��ݸ��ý�ͷ�ιܼ�ˮ���̶��ߣ��Ӹ�ҡ�ȣ���ȷ�IJ���˳��Ϊ
��4�����������У��٢۶��õ��������������÷ֱ�Ϊ
��5�����������У������С�ļ�ˮ�����̶��ߣ���ȡ�IJ��ȴ�ʩΪ
��6�����²����ᵼ����ҺŨ��ƫ�͵���
�����ձ����ܽ�ʱ��������Һ�彦��
�ڶ���ҡ�Ⱥ�����ҺҺ����ڿ̶��ߣ��ֵμ�����ˮ���̶���
������ƿʹ��ǰδ����
����ʹ�õ�����ƿ������0.5mol?L-1������ͭ��Һ��ϴ��
�ݶ��ݺ�Ӹ�ҡ�ȣ���װ��������ˮϴ����δ��ɵ��Լ�ƿ��
��û��ϴ���ܽ�ʱ�õ��ձ��Ͳ�������
��1����Ҫ����������Ϊ
12.5
12.5
g��2���ڸ�ʵ����Ҫ�õ�����ƿ��ʹ������ƿ�ĵ�һ����
����Ƿ�©ˮ
����Ƿ�©ˮ
����3����Դ����ƹ��������5���������裺����ʢ�е������ձ��м�����ˮʹ���ܽ⣻�ڼ���������ƿ�м�ˮ��Һ��ӽ��̶���1-2cm���۽�����ͭ��Һ�ز�����ע��500mL����ƿ�У������ձ��м�������ˮ��С��ϴ��2-3�κ���������ƿ�У��ݸ��ý�ͷ�ιܼ�ˮ���̶��ߣ��Ӹ�ҡ�ȣ���ȷ�IJ���˳��Ϊ
�٢ۢܢڢ�
�٢ۢܢڢ�
����4�����������У��٢۶��õ��������������÷ֱ�Ϊ
����
����
������
����
����5�����������У������С�ļ�ˮ�����̶��ߣ���ȡ�IJ��ȴ�ʩΪ
����Һ������������ƿϴˢ����������
����Һ������������ƿϴˢ����������
����6�����²����ᵼ����ҺŨ��ƫ�͵���
�٢ڢݢ�
�٢ڢݢ�
������ҺŨ����Ӱ�������
��
�������ձ����ܽ�ʱ��������Һ�彦��
�ڶ���ҡ�Ⱥ�����ҺҺ����ڿ̶��ߣ��ֵμ�����ˮ���̶���
������ƿʹ��ǰδ����
����ʹ�õ�����ƿ������0.5mol?L-1������ͭ��Һ��ϴ��
�ݶ��ݺ�Ӹ�ҡ�ȣ���װ��������ˮϴ����δ��ɵ��Լ�ƿ��
��û��ϴ���ܽ�ʱ�õ��ձ��Ͳ�������
��������1��û��480mL������ƿ����Ӧѡ��500mL����ƿ���ٸ�n=c��V���CuSO4�����ʵ�������CuSO4�����ʵ����뵨����ȣ�����m=nM������Ҫ������������
��2������ƿǰʹ��ǰ����Ƿ�©ˮ��
��3�����ݲ��������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ��������
��4���������ܽ�����в������������ǽ��裻ת�ƹ����в�������������������
��5�������Ƿ��������Һ��Ũ����Ӱ���жϣ�
��6������C=
�жϣ�������ʵ����ʵ���ƫ�����Һ�����ƫС���ᵼ��������Һ��Ũ��ƫ�ͣ�
��2������ƿǰʹ��ǰ����Ƿ�©ˮ��
��3�����ݲ��������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ��������
��4���������ܽ�����в������������ǽ��裻ת�ƹ����в�������������������
��5�������Ƿ��������Һ��Ũ����Ӱ���жϣ�
��6������C=
| n |
| V |
����⣺��1��û��480mL������ƿ����Ӧѡ��500mL����ƿ��������500mL 0.1mol?L-1��CuSO4��Һ������CuSO4�����ʵ���Ϊn=c��V=0.5L��0.100mol/L=0.0500mol��CuSO4�����ʵ����뵨����ȣ���Ҫ����������Ϊm��CuSO4?5H20��=0.05mol��250g/mol=12.5g��
�ʴ�Ϊ��12.5��
��2���ڸ�ʵ����Ҫ�õ�����ƿ��ʹ������ƿ�ĵ�һ���Ǽ���Ƿ�©ˮ��
�ʴ�Ϊ������Ƿ�©ˮ��
��3�����������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������ȷ�IJ���˳��Ϊ�٢ۢܢڢݣ�
�ʴ�Ϊ���٢ۢܢڢݣ�
��4�����ܽ�����в������������ǽ��裬ת�ƹ����в����������������������ԣ��٢۶��õ��������������÷ֱ�Ϊ���衢������
�ʴ�Ϊ�����衢������
��5�������С�ļ�ˮ�����̶��ߣ��������Ƶ���ҺŨ��ƫС�������Һ������������ƿϴˢ���������ƣ�
�ʴ�Ϊ������Һ������������ƿϴˢ���������ƣ�
��6�������ձ����ܽ�ʱ��������Һ�彦�������ʵ����ʵ���ƫС���������Ƶ���ҺŨ��ƫ�ͣ�
�ڶ���ҡ�Ⱥ�����ҺҺ����ڿ̶��ߣ��ֵμ�����ˮ���̶��ߣ���������̶��������Һ������������Һ�����ƫ�������Ƶ���ҺŨ��ƫ�ͣ�
������ƿʹ��ǰδ������ʵ����ʵ�������Һ����������䣬�������Ƶ���ҺŨ�Ȳ��䣻
����ʹ�õ�����ƿ������0.5mol?L-1������ͭ��Һ��ϴ�������ʵ����ʵ���ƫ�����Ե������Ƶ���ҺŨ��ƫ�ߣ�
�ݶ��ݺ�Ӹ�ҡ�ȣ���װ��������ˮϴ����δ��ɵ��Լ�ƿ�У��൱�ڶ���Һ������ϡ�ͣ��������Ƶ���ҺŨ��ƫ�ͣ�
��û��ϴ���ܽ�ʱ�õ��ձ��Ͳ����������ʵ����ʵ���ƫС���������Ƶ���ҺŨ��ƫ�ͣ�
���Իᵼ����ҺŨ��ƫ�͵��Ǣ٢ڢݢޣ�����ҺŨ����Ӱ����Ǣ�
�ʴ�Ϊ���٢ڢݢޣ��ۣ�
�ʴ�Ϊ��12.5��
��2���ڸ�ʵ����Ҫ�õ�����ƿ��ʹ������ƿ�ĵ�һ���Ǽ���Ƿ�©ˮ��
�ʴ�Ϊ������Ƿ�©ˮ��
��3�����������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������ȷ�IJ���˳��Ϊ�٢ۢܢڢݣ�
�ʴ�Ϊ���٢ۢܢڢݣ�
��4�����ܽ�����в������������ǽ��裬ת�ƹ����в����������������������ԣ��٢۶��õ��������������÷ֱ�Ϊ���衢������
�ʴ�Ϊ�����衢������
��5�������С�ļ�ˮ�����̶��ߣ��������Ƶ���ҺŨ��ƫС�������Һ������������ƿϴˢ���������ƣ�
�ʴ�Ϊ������Һ������������ƿϴˢ���������ƣ�
��6�������ձ����ܽ�ʱ��������Һ�彦�������ʵ����ʵ���ƫС���������Ƶ���ҺŨ��ƫ�ͣ�
�ڶ���ҡ�Ⱥ�����ҺҺ����ڿ̶��ߣ��ֵμ�����ˮ���̶��ߣ���������̶��������Һ������������Һ�����ƫ�������Ƶ���ҺŨ��ƫ�ͣ�
������ƿʹ��ǰδ������ʵ����ʵ�������Һ����������䣬�������Ƶ���ҺŨ�Ȳ��䣻
����ʹ�õ�����ƿ������0.5mol?L-1������ͭ��Һ��ϴ�������ʵ����ʵ���ƫ�����Ե������Ƶ���ҺŨ��ƫ�ߣ�
�ݶ��ݺ�Ӹ�ҡ�ȣ���װ��������ˮϴ����δ��ɵ��Լ�ƿ�У��൱�ڶ���Һ������ϡ�ͣ��������Ƶ���ҺŨ��ƫ�ͣ�
��û��ϴ���ܽ�ʱ�õ��ձ��Ͳ����������ʵ����ʵ���ƫС���������Ƶ���ҺŨ��ƫ�ͣ�
���Իᵼ����ҺŨ��ƫ�͵��Ǣ٢ڢݢޣ�����ҺŨ����Ӱ����Ǣ�
�ʴ�Ϊ���٢ڢݢޣ��ۣ�
���������⿼��һ�����ʵ���Ũ����Һ�����ƣ��漰���ƹ��̡���صļ��㣬��������ʱ����C=
�ж�������Һ��Ũ��ƫ����ƫ�ͣ�
| n |
| V |

��ϰ��ϵ�д�
�����Ŀ
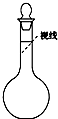 ʵ������Ҫ480mL 0.4mol?L-1��NaCl��Һ�������²������裺
ʵ������Ҫ480mL 0.4mol?L-1��NaCl��Һ�������²������裺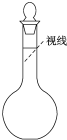 ʵ������Ҫ480mL 0.4mol?L-1��NaCl��Һ�������²������裺
ʵ������Ҫ480mL 0.4mol?L-1��NaCl��Һ�������²������裺