��Ŀ����
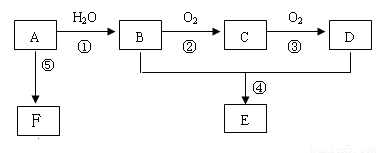
��֪AΪһ����̬��������Է�������С��30��1molA��ȫȼ�����ɵ�CO2��H2O���ʵ���֮��Ϊ1��1���м����C��������һ��Ҳ�ܸ����Ƶ�Cu��OH��2������Ӧ����ש��ɫ������CҲ�ܷ���������Ӧ��E����ζ��FΪһ�־ۺ������Ӧ����δд������

��ش��������⣺
��1������A�ͼ�����Բ��õļ�����������������ͽ��ۣ��У�
��2��A����B�ķ�Ӧ����Ϊ
��3������B���Ա�ֱ������ΪD����Ҫ������Լ���
��4����Ӧ�ܵĻ�ѧ����ʽΪ��
��5����ʵ�����л�õ�E���������Ǻ���B���ʺ�D���ʵĴֲ�Ʒ��Ҫ���ý�Ϊ������E���ʿ��Լ�����Լ���
��6����Ӧ�ݵĻ�ѧ����ʽΪ��
 ����Ӧ���ͣ�
����Ӧ���ͣ�

��ش��������⣺
��1������A�ͼ�����Բ��õļ�����������������ͽ��ۣ��У�
������ֱ�ͨ����ˮ��Һ�У���ʹ��ˮ��ɫ����������ϩ��������ֱ�ͨ�����Ը��������Һ�У���ʹ���Ը��������Һ��ɫ����������ϩ
������ֱ�ͨ����ˮ��Һ�У���ʹ��ˮ��ɫ����������ϩ��������ֱ�ͨ�����Ը��������Һ�У���ʹ���Ը��������Һ��ɫ����������ϩ
����2��A����B�ķ�Ӧ����Ϊ
�ӳɷ�Ӧ
�ӳɷ�Ӧ
��C�Ľṹ��ʽΪ��CH3CHO
CH3CHO
��B��D�й����ŵ����Ʒֱ����ǻ�
�ǻ�
���Ȼ�
�Ȼ�
����3������B���Ա�ֱ������ΪD����Ҫ������Լ���
������ػ��ظ������Һ
������ػ��ظ������Һ
����4����Ӧ�ܵĻ�ѧ����ʽΪ��
CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O
| ŨH2SO4 |
| �� |
CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O
����Ӧ���ͣ�| ŨH2SO4 |
| �� |
������Ӧ��ȡ����Ӧ
������Ӧ��ȡ����Ӧ
����5����ʵ�����л�õ�E���������Ǻ���B���ʺ�D���ʵĴֲ�Ʒ��Ҫ���ý�Ϊ������E���ʿ��Լ�����Լ���
����̼������Һ
����̼������Һ
����ط��������������Һ
��Һ
�������ƣ�����6����Ӧ�ݵĻ�ѧ����ʽΪ��


�Ӿ۷�Ӧ
�Ӿ۷�Ӧ
��������AΪһ����̬��������Է�������С��30��1molA��ȫȼ�����ɵ�CO2��H2O���ʵ���֮��Ϊ1��1����A��̼��ԭ�Ӹ�����Ϊ1��2��
��A�ķ���ʽΪ��CH2��x������Է�������С��30����XΪ2��
���Ը���̬��Ϊ��ϩ����ṹ��ʽΪCH2=CH2��
A��ˮ�����ӳɷ�Ӧ�����Ҵ�����B�Ľṹ��ʽΪCH3CH2OH��
�Ҵ�������������ȩ����CΪCH3CHO��
C�������������ᣬ��DΪCH3COOH��
�Ҵ������ᷢ��������Ӧ����������������E�Ľṹ��ʽΪCH3COOCH2CH3��
FΪһ�־ۺ����F�Ľṹ��ʽΪ ��
��
��A�ķ���ʽΪ��CH2��x������Է�������С��30����XΪ2��
���Ը���̬��Ϊ��ϩ����ṹ��ʽΪCH2=CH2��
A��ˮ�����ӳɷ�Ӧ�����Ҵ�����B�Ľṹ��ʽΪCH3CH2OH��
�Ҵ�������������ȩ����CΪCH3CHO��
C�������������ᣬ��DΪCH3COOH��
�Ҵ������ᷢ��������Ӧ����������������E�Ľṹ��ʽΪCH3COOCH2CH3��
FΪһ�־ۺ����F�Ľṹ��ʽΪ
 ��
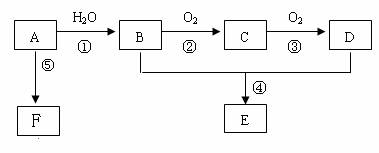
������⣺AΪһ����̬��������Է�������С��30��1molA��ȫȼ�����ɵ�CO2��H2O���ʵ���֮��Ϊ1��1����A��̼��ԭ�Ӹ�����Ϊ1��2��
��A�ķ���ʽΪ��CH2��x������Է�������С��30����XΪ2��
���Ը���̬��Ϊ��ϩ����ṹ��ʽΪCH2=CH2��
A��ˮ�����ӳɷ�Ӧ�����Ҵ�����B�Ľṹ��ʽΪCH3CH2OH��
�Ҵ�������������ȩ����CΪCH3CHO��
C�������������ᣬ��DΪCH3COOH��
�Ҵ������ᷢ��������Ӧ����������������E�Ľṹ��ʽΪCH3COOCH2CH3��
FΪһ�־ۺ����F�Ľṹ��ʽΪ ��
��
��1����ϩ����̼̼˫�������������ʽϻ��ã��ܺ���ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ���ܱ����Ը��������Һ������ʹ���Ը��������Һ��ɫ�����鲻��̼̼˫�������ʽ��ȶ�������ǿ��ǿ�Ӧ�����ܱ����Ը��������Һ��������������������ϩ�ķ����У�������ֱ�ͨ����ˮ��Һ�У���ʹ��ˮ��ɫ����������ϩ��������ֱ�ͨ�����Ը��������Һ�У���ʹ���Ը��������Һ��ɫ����������ϩ��
�ʴ�Ϊ��������ֱ�ͨ����ˮ��Һ�У���ʹ��ˮ��ɫ����������ϩ��������ֱ�ͨ�����Ը��������Һ�У���ʹ���Ը��������Һ��ɫ����������ϩ��
��2��A��ˮ�����ӳɷ�Ӧ����B�����Ը÷�Ӧ���ڼӳɷ�Ӧ��ͨ�����Ϸ���֪��C�Ľṹ��ʽΪ��CH3CHO��B��D�й����ŵ����Ʒֱ��� �ǻ����Ȼ���
�ʴ�Ϊ���ӳɷ�Ӧ��CH3CHO���ǻ����Ȼ���
��3���Ҵ��ܱ�ǿ�������������Ը�����ػ��ظ���������������ᣬ
�ʴ�Ϊ��������ػ��ظ������Һ��
��4���ڼ��ȡ�Ũ���������������£��Ҵ������ᷴӦ��������������ˮ����Ӧ����ʽΪ��
CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O���÷�Ӧ����������Ӧ��ȡ����Ӧ��
�ʴ�Ϊ��CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��������Ӧ��ȡ����Ӧ��
��5�����������к���������Ҵ��������̼���Ʒ�Ӧ���������ƺͶ�����̼��ˮ���Ҵ���̼���ƻ��ܣ����������ͱ���̼���Ʋ����ܣ�����Ҫ��ȥ���������е�������Ҵ�ʹ�õ��Լ��DZ���̼������Һ�����÷�Һ�ķ������룬
�ʴ�Ϊ������̼������Һ����Һ��
��6���ڴ��������£���ϩ�����Ӿ۷�Ӧ���ɾ���ϩ����Ӧ����ʽΪ�� ���÷�Ӧ���ڼӾ۷�Ӧ��
���÷�Ӧ���ڼӾ۷�Ӧ��
�ʴ�Ϊ�� ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ��
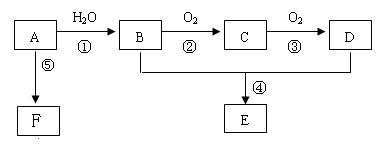
��A�ķ���ʽΪ��CH2��x������Է�������С��30����XΪ2��
���Ը���̬��Ϊ��ϩ����ṹ��ʽΪCH2=CH2��
A��ˮ�����ӳɷ�Ӧ�����Ҵ�����B�Ľṹ��ʽΪCH3CH2OH��
�Ҵ�������������ȩ����CΪCH3CHO��
C�������������ᣬ��DΪCH3COOH��
�Ҵ������ᷢ��������Ӧ����������������E�Ľṹ��ʽΪCH3COOCH2CH3��
FΪһ�־ۺ����F�Ľṹ��ʽΪ
 ��
����1����ϩ����̼̼˫�������������ʽϻ��ã��ܺ���ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ���ܱ����Ը��������Һ������ʹ���Ը��������Һ��ɫ�����鲻��̼̼˫�������ʽ��ȶ�������ǿ��ǿ�Ӧ�����ܱ����Ը��������Һ��������������������ϩ�ķ����У�������ֱ�ͨ����ˮ��Һ�У���ʹ��ˮ��ɫ����������ϩ��������ֱ�ͨ�����Ը��������Һ�У���ʹ���Ը��������Һ��ɫ����������ϩ��
�ʴ�Ϊ��������ֱ�ͨ����ˮ��Һ�У���ʹ��ˮ��ɫ����������ϩ��������ֱ�ͨ�����Ը��������Һ�У���ʹ���Ը��������Һ��ɫ����������ϩ��
��2��A��ˮ�����ӳɷ�Ӧ����B�����Ը÷�Ӧ���ڼӳɷ�Ӧ��ͨ�����Ϸ���֪��C�Ľṹ��ʽΪ��CH3CHO��B��D�й����ŵ����Ʒֱ��� �ǻ����Ȼ���
�ʴ�Ϊ���ӳɷ�Ӧ��CH3CHO���ǻ����Ȼ���
��3���Ҵ��ܱ�ǿ�������������Ը�����ػ��ظ���������������ᣬ
�ʴ�Ϊ��������ػ��ظ������Һ��
��4���ڼ��ȡ�Ũ���������������£��Ҵ������ᷴӦ��������������ˮ����Ӧ����ʽΪ��
CH3CH2OH+CH3COOH
| ŨH2SO4 |
| �� |
�ʴ�Ϊ��CH3CH2OH+CH3COOH
| ŨH2SO4 |
| �� |
��5�����������к���������Ҵ��������̼���Ʒ�Ӧ���������ƺͶ�����̼��ˮ���Ҵ���̼���ƻ��ܣ����������ͱ���̼���Ʋ����ܣ�����Ҫ��ȥ���������е�������Ҵ�ʹ�õ��Լ��DZ���̼������Һ�����÷�Һ�ķ������룬
�ʴ�Ϊ������̼������Һ����Һ��
��6���ڴ��������£���ϩ�����Ӿ۷�Ӧ���ɾ���ϩ����Ӧ����ʽΪ��
 ���÷�Ӧ���ڼӾ۷�Ӧ��
���÷�Ӧ���ڼӾ۷�Ӧ���ʴ�Ϊ��
 ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ�����������⿼�����л�����ƶϼ����ʼ��飬��ȷ�ƶ�A�ǽⱾ��ؼ��������������ϵķ�������������Ը�����ؾ���ǿ�����ԣ��ܰ�ϩ�����ǻ�λ�ڱ��ϵĴ���ȩ�������ӱ�����̼ԭ���Ϻ�����ԭ�ӵı���ͬϵ�������������ᣮ

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 �� ��
�� �� ��
��  _________
_________ ����ط������������
����ط������������  __�������ƣ���
__�������ƣ���