题目内容
( 12分)Ⅰ.(1)在一密闭的2L的容器里充入8mol SO2和4mol 18O2,在一定条件下开始反应:2SO2(g)+O2(g) 2SO3(g)2min末测得容器中有7.2mol SO2。试回答:
2SO3(g)2min末测得容器中有7.2mol SO2。试回答:
①反应后18O原子存在于哪些物质中 ;
② 2min末SO3的浓度________________________;
③用O2的浓度变化表示该时间段内的化学反应速率_______________________。
Ⅱ.某化学反应2A (g) B(g)+D(g)在3种不同条件下进行,B和D的起始浓度为0,反应物A的浓度(mol/L)随反应时间(min)的变化情况如下表:
B(g)+D(g)在3种不同条件下进行,B和D的起始浓度为0,反应物A的浓度(mol/L)随反应时间(min)的变化情况如下表:
| 实验序号 |  | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
| 1 | 800℃ | 1.0 | 0.80 | 0.67 | 0.57 | 0.50 | 0.50 | 0.50 |
| 2 | 800℃ | C2 | 0.92 | 0.75 | 0.63 | 0.60 | 0.60 | 0.60 |
| 3 | 820℃ | 1.0 | 0.40 | 0.25 | 0.20 | 0.20 | 0.20 | 0.20 |
(1) 实验1达到平衡的时间是__________min,C2_____1.0 min·L-1(填“<”“>”或“=”)。
(2)实验3比实验1的反应速率_________(填“快”或“慢”),原因是___________________________________________________________________________。
(3) 如果2A (g) B(g)+D(g)是一个吸热反应,那么实验3与实验1相比,在相同体积时___________吸收的热量多,理由是___________________________________________。
Ⅰ
Ⅱ(1)40(1分),>(1分)(2)快(1分), 实验3比实验1的温度高,反应速率快(2分)(3)实验3中A的转化率大些(分解的A多些),正反应吸收热量多。(2分)
解析

 嫦娥三号探测器已于2013年12月2日凌晨1:30分在四川省西昌卫星发射中心使用长征三号乙增强型运载火箭发射成功.火箭的一子级和二子级使用偏二甲肼和N2O4作为推进剂,反应式为(CH3)2NNH2+2N2O4═2CO2+4H2O+3N2,三子级则使用效能更高的液氢(H2)和液氧(O2).下列说法正确的是( )
嫦娥三号探测器已于2013年12月2日凌晨1:30分在四川省西昌卫星发射中心使用长征三号乙增强型运载火箭发射成功.火箭的一子级和二子级使用偏二甲肼和N2O4作为推进剂,反应式为(CH3)2NNH2+2N2O4═2CO2+4H2O+3N2,三子级则使用效能更高的液氢(H2)和液氧(O2).下列说法正确的是( )| A、N2O4在反应中被氧化 | B、(CH3)2NNH2具有还原性 | C、反应中1mol N2O4得到4mol e- | D、液氢与液氧的反应中,液氢与液氧的体积比为 2:1 |
(共12分)配制250mL 1.0mol/L NaOH溶液,请回答下列问题:
⑴在下列仪器中:A 托盘天平 B 量筒 C烧杯 D 玻璃棒 E 漏斗 F 500mL容量瓶
G 药匙 H 250mL容量瓶 I 胶头滴管 J 坩埚
需要用到的仪器有
⑵所需NaOH固体质量为 克
⑶配制的实验步骤如下:
①计算 ②称量 ③溶解 ④转移、洗涤 ⑤定容 ⑥摇匀
其中第③、④、⑤步实验操作都要用到玻璃棒,作用分别是 , ,
。
⑷在容量瓶内确定溶液体积的过程中,完成后期加入少量水的做法是
;
下列各项中,可能导致实际浓度偏高的是 (填标号)
| A.在天平托盘上垫纸,将NaOH放在纸上称量 |
| B.NaOH溶解时放出大量的热,未冷却立即配制溶液 |
| C.溶解NaOH固体之后的烧杯未洗涤 |
| D.向容量瓶中转移液体时不慎洒出 |
⑹将取出的10mL溶液加水稀释到100mL,稀释后溶液中N
 aOH的物质的量浓度为 。
aOH的物质的量浓度为 。 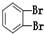 ⑨235U ⑩
⑨235U ⑩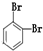
 (12)
(12)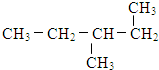
 四种分子中,碳原子为sp2杂化的分子是_______________________________________________。
四种分子中,碳原子为sp2杂化的分子是_______________________________________________。 键有_______mol和形成
键有_______mol和形成 键有_______mol。
键有_______mol。