��Ŀ����
��7�֣��������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ����
�ش��������⣺
��1����ԭ�ӵĵ����Ų�ʽ����������������������������������������������
��2��������Ӧ���������������� �����ţ���
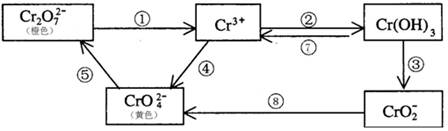
��3����ҵ�ϴ�����Cr2O72-�ķ�ˮʱ��һ�㽫�綾��Cr2O72-ת��ΪCr3+,д����̼Ϊ����������������������NaCl��Cr2O72-�����Է�ˮ��д���缫��Ӧ����Һ�н��еķ�Ӧ�����ӷ���ʽ����������������������������������������������������
����Һ����������������������������������������������
��4����Ӧ���ǿ��淴Ӧ����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��д���÷�Ӧ�����ӷ���ʽ��������������������������������������������������
��5����֪Ag2CrO4��AgCl��Ksp�ֱ�Ϊ9.0��10��12��1.56��10��10��������ͬŨ�ȵ�Na2CrO4��NaCl�Ļ����Һ����μ�����������Һ���������ɵij�������������
����:

��ϰ��ϵ�д�
�����Ŀ
 Cr2O72-+H2O
Cr2O72-+H2O