��Ŀ����
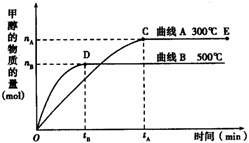 ����ͼ��ʾ��һ�������£������Ϊ3 L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO(g) + 2H2(g)
����ͼ��ʾ��һ�������£������Ϊ3 L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO(g) + 2H2(g) ![]() CH3OH(g)
CH3OH(g)
�������淴Ӧ����ӦΪ___________��Ӧ������ȡ������ȡ�����
��500�棬�ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2) =___________________________����nB��tB��ʾ����
�Դ���E�����ϵ���ı��������ʹƽ��������Ӧ�����ƶ�ʱ�������йظ���ϵ��˵����ȷ����______________________������ĸ����
A��H2��ת����һ������ B��v��һ������v��һ����С
C��CH3OH����������һ������ D��v��һ��С��v��
��1�� ���� ��3�֣�
��2��2nB/3tBmol/��L��min����3�֣�
��3��D��3�֣�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ