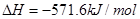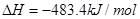��Ŀ����
��101kPa��25��ʱ���Ȼ�ѧ����ʽ��H2(g) + 1/2O2(g) =H2O(g)����H=-241.8kJ/mol��H2(g)+ 1/2O2(g) =H2O(1)����H=-285.8kJ/mol������˵���д�����ǣ� ( )
A��H2ȼ������1molH2O(g)ʱ���ų�241.8kJ������
B��H2��ȼ����Ϊ285.8kJ
C��O2ǰ���1/2��ʾ�μӷ�Ӧ��O2�����ʵ���
D��1molҺ̬ˮ���ˮ����ʱ����44kJ������
B
����:

��ϰ��ϵ�д�
�����Ŀ
��101kPa��25��ʱ���йط�Ӧ���Ȼ�ѧ����ʽ���£�
C��s��+
O2��g��=CO��g����H1=-111kJ?mol-1
H2��g��+
O2��g��=H2O��g����H2=-242kJ?mol-1
C��s��+O2��g��=CO2��g����H3=-394kJ?mol-1
��ͬ�����£�CO��g��+H2O��g��=H2��g��+CO2��g����H�����H�ǣ�������
C��s��+
| 1 |
| 2 |
H2��g��+
| 1 |
| 2 |
C��s��+O2��g��=CO2��g����H3=-394kJ?mol-1
��ͬ�����£�CO��g��+H2O��g��=H2��g��+CO2��g����H�����H�ǣ�������
| A��-41kJ?mol-1 |
| B��+41kJ?mol-1 |
| C��-152kJ?mol-1 |
| D��-263kJ?mol-1 |

 =
=
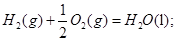
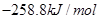
 ��
��
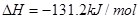
 ȼ���ȵĻ�ѧ����ʽΪ��
ȼ���ȵĻ�ѧ����ʽΪ��