��Ŀ����
��15�֣�MnO2��������Һ�о���ǿ�����ԣ��ɱ���ԭΪMn2+��������H2O2�ķֽ�������õĴ�Ч����ij��ȤС��ͨ��ʵ���о�MnO2�����ʡ�
��1����С�����������3����������֤MnO2�������ԣ����е�������������������
A����MnO2������뵽FeSO4��Һ�У��ټ���KSCN��Һ���۲���Һ�Ƿ���
B����MnO2������뵽FeCl3��Һ�У��ټ���KSCN��Һ���۲���Һ�Ƿ���
C����MnO2������뵽ϡ�����У��۲��Ƿ��л���ɫ��������
��2����С��Ϊ�о��ڲ�ͬ����Ե���Һ��MnO2���������������ǿ���KI��Һ��Ũ�Ⱥ�MnO2�����������ͬ���㶨ʵ���¶���298K��������¶Ա����顣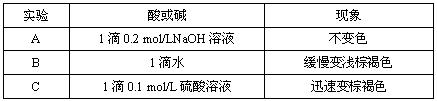
��С��������Ա�ʵ���У����Եó��Ľ���������������������������������������
д�������������£�MnO2����I�������ӷ���ʽ���������������������������� ��
��3����̽��MnO2�Ĵ�Ч������Ҫ��30%��H2O2��Һ���ܶȽ���Ϊ1g/cm3������Ũ��3%��H2O2��Һ���ܶȽ���Ϊ1g/cm3��100mL�������Ʒ����ǣ�����Ͳ��ȡ�� mL30%H2O2��Һ�����������������������ƣ��У��ټ���һ������ˮ��������ȡ�
��4����ʵ��ʱ��ijͬѧ��1��KI��Һ����뵽������5 mL 3%��H2O2��Һ�У����ֲ����˴������ݡ���С����ĵ�KI��H2O2�ɷ������·�Ӧ��2KI��H2O2 ��KOH ��I2����Ϊ�п����Ƿ�Ӧ����I2���˴�H2O2�ֽ�����á������һ����ʵ��֤���ü����Ƿ���ȷ��������������������������������������������������������������
��5��ʵ�����ö������̺�Ũ������ȡ��������������������в���Ҫ�õ����� ������ţ���
a������©�� b��Բ����ƿ c���¶ȼ� d���ƾ��� e��ϴ��ƿ f���ձ�
��1��A ��2�֣�
��2������Խǿ��MnO2������Խǿ ��2�֣�
MnO2 + 2I��+ 4H+ ��Mn2+��I2 + 2H2O ��3�֣�
��3��10.0 �ձ� ����2�֣�
��4��ȡ5mL3%��H2O2��Һ���Թ��У�����1�ε�ˮ���۲��Ƿ��д������ݲ���������˵��������ȷ����֮���費��ȷ ��2�֣�
��2�֣�
��5��a��c ��2�֣�
����

��15�֣�
MnO2��������Һ�о���ǿ�����ԣ��ɱ���ԭΪMn2+��������H2O2�ķֽ�������õĴ�Ч����ij��ȤС��ͨ��ʵ���о�MnO2������
��1����С�����������4����������֤MnO2�������ԣ����е�������������������
| A����MnO2������뵽FeSO4��Һ�У��ټ���KSCN��Һ���۲���Һ�Ƿ��� |
| B����MnO2������뵽FeCl3��Һ�У��ټ���KSCN��Һ���۲���Һ�Ƿ��� |
| C����MnO2������뵽Na2SO3��Һ�У��ټ���BaCl2�۲��Ƿ��а�ɫ�������� |
| D����MnO2������뵽ϡ�����У��۲��Ƿ��л���ɫ�������� |
| ʵ�� | ���� | ���� |
| A | 1��0.2mol/LNaOH��Һ | ����ɫ |
| B | 1��ˮ | ������dz�غ�ɫ |
| C | 1��0.1mol/L������Һ | Ѹ�ٱ��غ�ɫ |
д�������������£�MnO2����I�������ӷ���ʽ���������������������������� ��
��3����̽��MnO2�Ĵ�Ч������Ҫ��30%��H2O2��Һ���ܶȽ���Ϊ1g/cm3������Ũ��3%��H2O2��Һ���ܶȽ���Ϊ1g/cm3��100mL�������Ʒ����ǣ�����Ͳ��ȡ������mL30%H2O2��Һ�����������������������ƣ��У��ټ���һ������ˮ��������ȡ�
��4����ʵ��ʱ��ijͬѧ��1��KI��Һ����뵽������5mL3%��H2O2��Һ�У����ֲ����˴������ݡ���С����ĵ�KI��H2O2�ɷ������·�Ӧ��2KI��H2O2��KOH��I2����Ϊ�п����Ƿ�Ӧ����I2���˴�H2O2�ֽ�����á������һ����ʵ��֤���ü����Ƿ���ȷ��������������������������������������������������������������������������
������������������������������������������������������������������������������
��5��ʵ�����ö������̺�Ũ������ȡ������������������Ϊ�÷�Ӧ�ķ�Ӧ�������� ������ţ���
������������
 ��������
�������� ������
������ ����
����
��������������A �� B C D
��6���������̿����������ɵ�أ�����ܷ�ӦΪ��Zn��2MnO2��2NH4����Zn2����Mn2O3��2NH3��H2O�����������ĵ缫��ӦʽΪ �������� ��
 2ZnO��2SO2 2C��O2
2ZnO��2SO2 2C��O2