��Ŀ����
����Ŀ����֪���л���A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ1��ʾ��
��1��D�����й����ŵ������� �� �߷��ӻ�����E����������
��2����Ӧ�ڵĻ�ѧ����ʽ������Ӧ�ܵĻ�ѧ����ʽ�� ��
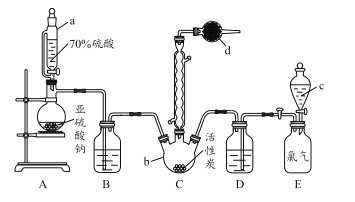
��3��ijͬѧ����ͼ2��ʾ��ʵ��װ����ȡ��������������ʵ��������Թܼ����ϲ�Ϊ���ġ�������ˮ����״Һ�壮
��ʵ�鿪ʼʱ���Թܼ��еĵ��ܲ�����Һ���µ�ԭ������
������ʵ���б���̼������Һ����������
����ʵ��������B��D�Ʊ�����������ʵ���У�����1mol B��1mol D��ַ�Ӧ����������1mol ����������ԭ���� ��
���𰸡�
��1���Ȼ�,����ϩ
��2��2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O,CH3COOH+C2H5OH
2CH3CHO+2H2O,CH3COOH+C2H5OH ![]() CH3COOC2H5+H2O
CH3COOC2H5+H2O
��3����ֹ��Һ����,�ܽ��Ҵ����������ᡢ�������������ܽ��,���淴Ӧ
���������⣺A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ�õ�BΪCH3CH2OH���Ҵ������ᷢ��������Ӧ������������������DΪCH3COOH���Ҵ���������������ȩ����ȩ�������������ᣬ����CΪCH3CHO��A�����Ӿ۷�Ӧ����E��EΪ ![]() ����1��DΪ���ᣬ���������Ȼ���E�Ǹ߷��ӻ��������ϩ�����Դ��ǣ��Ȼ�������ϩ��
����1��DΪ���ᣬ���������Ȼ���E�Ǹ߷��ӻ��������ϩ�����Դ��ǣ��Ȼ�������ϩ��
��2���Ҵ�������������Ӧ������ȩ����Ӧ����ʽΪ2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
������Ҵ�����������Ӧ����������������Ӧ����ʽΪCH3COOH+C2H5OH ![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
���Դ��ǣ�2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O��CH3COOH+C2H5OH
2CH3CHO+2H2O��CH3COOH+C2H5OH ![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
��3���ٻӷ��������ᡢ�Ҵ�����������ˮ�����������������Һ���»ᵼ�µ�����Ϊ�˷�ֹ�����������ܲ�������Һ���£����Դ��ǣ���ֹ��Һ������
���Ҵ������ᶼ�ӷ����Ʊ����������������Ҵ������ᣬͨ���ñ���̼������Һ�������ᣬ�ܽ��Ҵ�����Ӧ���ӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У��ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
���Դ��ǣ��ܽ��Ҵ����������ᡢ�������������ܽ�ȣ�
�۸÷�Ӧ�ǿ��淴Ӧ���·�Ӧ�ﲻ����ȫת��Ϊ��������Բ�������1mol ����������
���Դ��ǣ����淴Ӧ��