��Ŀ����
��10�֣�ij��ѧ��ȤС��������ͼװ�ö�ij��п��Ʒ���д��ȼ�⡣����д����ʵ�鱨�档
(1)ʵ��Ŀ�ģ� ��
(2)ʵ�鲽�裺
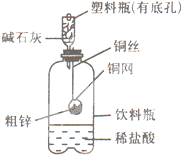
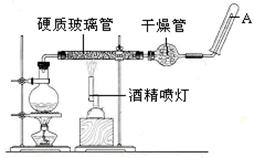
�ٳ�ȡ10��0 g��п����ͭ���У���ͼʾװ����װ�Ƶ�������ҩƷ������Ϊ120��0 g��
�ڽ�ͭ������������ϡ�����У��йط�Ӧ�Ļ�ѧ����ʽΪ ��
�۷�Ӧ��ȫ�Ƶ�װ��������Ϊ119��8 g�����п�Ĵ���Ϊ ��
(3)����̽����(��֪��ʯ��Ϊ��Na()H��CaO�Ļ����)
�ٸ�ʵ���м�ʯ�ҵ������� �������ü�ʯ�ң�������õĴ�п���Ƚ� ��(�ƫ����ƫС������Ӱ�족)��
��������п����ʯ��ʯ��ԭʵ�鷽�� (��ܡ����ܡ�)����ʯ��ʯ��Ʒ���ȵIJⶨ�������� ��
(1)ʵ��Ŀ�ģ� ��
(2)ʵ�鲽�裺

�ٳ�ȡ10��0 g��п����ͭ���У���ͼʾװ����װ�Ƶ�������ҩƷ������Ϊ120��0 g��
�ڽ�ͭ������������ϡ�����У��йط�Ӧ�Ļ�ѧ����ʽΪ ��
�۷�Ӧ��ȫ�Ƶ�װ��������Ϊ119��8 g�����п�Ĵ���Ϊ ��
(3)����̽����(��֪��ʯ��Ϊ��Na()H��CaO�Ļ����)
�ٸ�ʵ���м�ʯ�ҵ������� �������ü�ʯ�ң�������õĴ�п���Ƚ� ��(�ƫ����ƫС������Ӱ�족)��
��������п����ʯ��ʯ��ԭʵ�鷽�� (��ܡ����ܡ�)����ʯ��ʯ��Ʒ���ȵIJⶨ�������� ��
��1���ⶨ��п��Ʒ��п�������������ȣ���2�֣�
��2����Zn+2HCl=ZnCl2+H2����2�֣� ��65%��2�֣�
��3��������ˮ�������Ȼ������壨1�֣� ƫ��1�֣�
�ڲ��ܣ�1�֣� ��ʯ�������շ�Ӧ���ɵ�CO2��1�֣�
��2����Zn+2HCl=ZnCl2+H2����2�֣� ��65%��2�֣�
��3��������ˮ�������Ȼ������壨1�֣� ƫ��1�֣�
�ڲ��ܣ�1�֣� ��ʯ�������շ�Ӧ���ɵ�CO2��1�֣�
��1�������Ƕ�ij��п��Ʒ���д��ȼ�⣬����ʵ��Ŀ���Dzⶨ��п��Ʒ��п�������������ȣ���
��2����п�ǻ��õĽ����������ᷴӦ��������������ʽΪZn+2HCl=ZnCl2+H2����
�ڼ������������Dz�������������������0.2g����������п��6.5g�����Դ�п�Ĵ���Ϊ6.5g��10.0g��100����65����
��3�������ڲ����������к���ˮ�������Ȼ��⣬���Լ�ʯ�ҵ�����������ˮ�������Ȼ������塣���û�м�ʯ�ң������������ƫ�࣬�ⶨ���ƫ��
��̼��ƺ����ᷴӦ���ɵ�CO2���ܱ���ʯ�����գ����Բ��ܡ�
��2����п�ǻ��õĽ����������ᷴӦ��������������ʽΪZn+2HCl=ZnCl2+H2����
�ڼ������������Dz�������������������0.2g����������п��6.5g�����Դ�п�Ĵ���Ϊ6.5g��10.0g��100����65����
��3�������ڲ����������к���ˮ�������Ȼ��⣬���Լ�ʯ�ҵ�����������ˮ�������Ȼ������塣���û�м�ʯ�ң������������ƫ�࣬�ⶨ���ƫ��
��̼��ƺ����ᷴӦ���ɵ�CO2���ܱ���ʯ�����գ����Բ��ܡ�

��ϰ��ϵ�д�
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ
 ��
��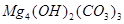 ��
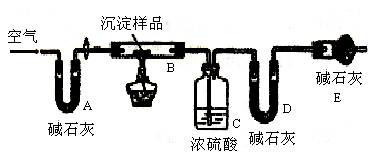
�� �ȡ��������һ���ⶨ��ʽ̼��þ��ɵ�ʵ�鷽��������
�ȡ��������һ���ⶨ��ʽ̼��þ��ɵ�ʵ�鷽��������