��Ŀ����
 ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ���������1��ʵ������ȡ��ϩ�Ļ�ѧ����ʽ
CH3CH2OH
CH2=CH2��+H2O
| Ũ���� |
| 170�� |
CH3CH2OH
CH2=CH2��+H2O
����Ӧ����Ϊ| Ũ���� |
| 170�� |
��ȥ��Ӧ
��ȥ��Ӧ
����2����װ�ÿ�ʢ�ŵ��Լ��Ǣ�
B
B
����C
C
����B
B
��A
A
�����������й��Լ����������ո��ڣ�A������KMnO4 B��Ʒ����Һ C��NaOH��Һ D��ŨH2SO4
��3����˵����������������ڵ�������
װ�â��е�Ʒ����Һ��ɫ
װ�â��е�Ʒ����Һ��ɫ
����4��װ�â��п�ʼ��Ӧʱ�����ӷ���ʽ��
SO2+2OH-=SO32-+H2O
SO2+2OH-=SO32-+H2O
����5��ʹ��װ�â��Ŀ����
����SO2�Ƿ����
����SO2�Ƿ����
����6��ȷ֤������ϩ������
���е�Ʒ�첻��ɫ�����еĸ��������Һ��ɫ
���е�Ʒ�첻��ɫ�����еĸ��������Һ��ɫ
����������1��ʵ�����Ʊ���ϩ���õ�ԭ��Ϊ�Ҵ���Ũ��������������ˮ������Ӧ�����Ǽ��ȵ�170�棻
��2�����ֲ��������ʱ��Ӧ�����Ⱥ�˳��
��3������������Ư��Ʒ�죻
��4���������������ն�������
��5��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���
��6��������������������������Һ���õ�Ϊ��ϩ��
��2�����ֲ��������ʱ��Ӧ�����Ⱥ�˳��
��3������������Ư��Ʒ�죻
��4���������������ն�������
��5��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���
��6��������������������������Һ���õ�Ϊ��ϩ��
����⣺��1�������Ҵ���Ũ����Ĵ������·�����������ˮ��ȡ��ϩ���Ҵ���������ȥ��Ӧ���ʴ�Ϊ��CH3CH2OH
CH2=CH2��+H2O����ȥ��Ӧ��
��2���������������Ʒ����Һ��������ϩ�ø������������Һ����ϩ�Ͷ���������ʹ�������������Һ��ɫ�������ȼ����������Ȼ�������ϩ��ͬ�ڼ�����ϩ֮ǰ��NaOH��Һ����SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫ������ϩ����װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ��II�Թ�װ��NaOH��Һ��ȥSO2��װ��III�Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ��IV ͨ���������������Һ��ɫ������ϩ��
�ʴ�Ϊ��B��C��B��A��
��3��װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2���ʴ�Ϊ������Ʒ����Һ��ɫ��
��4��װ�â��Թ�װ��NaOH��Һ��ȥSO2��SO2+2NaOH=Na2SO3+H2O���ʴ�Ϊ��SO2+2OH-=SO32-+H2O��
��5��װ�â��Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ����ʴ�Ϊ������SO2�Ƿ������
��6��װ�â�ͨ���������������Һ��ɫ������ϩ���ʴ�Ϊ�����е�Ʒ�첻��ɫ�����еĸ��������Һ��ɫ��
| Ũ���� |
| 170�� |
��2���������������Ʒ����Һ��������ϩ�ø������������Һ����ϩ�Ͷ���������ʹ�������������Һ��ɫ�������ȼ����������Ȼ�������ϩ��ͬ�ڼ�����ϩ֮ǰ��NaOH��Һ����SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫ������ϩ����װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ��II�Թ�װ��NaOH��Һ��ȥSO2��װ��III�Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ��IV ͨ���������������Һ��ɫ������ϩ��
�ʴ�Ϊ��B��C��B��A��
��3��װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2���ʴ�Ϊ������Ʒ����Һ��ɫ��
��4��װ�â��Թ�װ��NaOH��Һ��ȥSO2��SO2+2NaOH=Na2SO3+H2O���ʴ�Ϊ��SO2+2OH-=SO32-+H2O��
��5��װ�â��Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ����ʴ�Ϊ������SO2�Ƿ������
��6��װ�â�ͨ���������������Һ��ɫ������ϩ���ʴ�Ϊ�����е�Ʒ�첻��ɫ�����еĸ��������Һ��ɫ��
���������⿼������ϩ��ʵ�����Ʒ��Լ�����ļ��飬ע��ж��ֲ��������ʱ��Ӧ�����Ⱥ�˳������ؼ�������������������������ϩ���ǽ����Ĺؼ���

��ϰ��ϵ�д�
 �������ϵ�д�
�������ϵ�д�
�����Ŀ
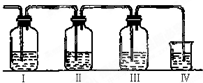 ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������I����װ�ÿ�ʢ�ŵ��Լ��ֱ��ǣ�I��Ʒ����Һ��NaOH��Һ��Ʒ����Һ��������KMnO4
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������I����װ�ÿ�ʢ�ŵ��Լ��ֱ��ǣ�I��Ʒ����Һ��NaOH��Һ��Ʒ����Һ��������KMnO4
