��Ŀ����
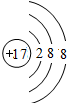
A��B��C��D����Ԫ�ض��Ƕ�����Ԫ�أ�AԪ�ص����Ӿ��л�ɫ����ɫ��Ӧ��BԪ�ص����ӽṹ��Ne������ͬ�ĵ��Ӳ��Ų���5.8 g B����������ǡ������100 mL 2 mol��L-1������ȫ��Ӧ��Bԭ�Ӻ�������������������ȡ�H2��C������ȼ�ղ�����ɫ���档DԪ��ԭ�ӵĵ��Ӳ�ṹ�У������������Ǵ�����������3���������������������������:
(1)Ԫ��Cλ�ڵ�________���ڵ�________�壬�������������Ļ�ѧʽΪ________��
(2)AԪ����________��BԪ����________��DԪ����________��
(3)A��D�γ��ȶ�������Ļ�ѧʽ��________���û������д��ڻ�ѧ������Ϊ________���жϸû������ڿ������Ƿ���ʵļ�����
________________________________________��
(4)CԪ�صĵ����ж�������A������������Ӧ��ˮ�������Һ���գ������ӷ���ʽΪ______________________________________________________________________________��
(1)3 ��A Cl2O7
(2)Na Mg O
(3)Na2O2 ���Ӽ� ��������ɫ�Ƿ���
(4)Cl2+2OH-====Cl-+ClO-+H2O
����:
����������AԪ������Ԫ�أ�������Ŀ���������ͼ��㣬BԪ����þԪ�أ�����ʵ������CԪ������Ԫ�أ�λ��Ԫ�����ڱ��ĵ�3���ڵڢ�A�壻A��DԪ�����γɵ��ȶ��������ǹ������ƣ��жϹ��������Ƿ���ʣ�ֻҪ��������ɫ�Ƿ��ף�����������������Һ��Ӧ�ķ���ʽΪCl2+2OH-====Cl-+ClO-+H2O��

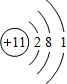
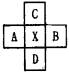
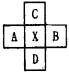
 ��B��C���γ����ӻ�����
��B��C���γ����ӻ����� ��B��C���γ����ӻ�����
��B��C���γ����ӻ�����