��Ŀ����
����A��B����������֪A�ķ���ʽΪC5Hm����B�����ʽΪC5Hn��m��n��Ϊ������������ش��������⣺��1�����й�����A����B��˵������ȷ����______������ţ���
a����A����B���ܻ�Ϊͬϵ��
b����A����B���ܻ�Ϊͬ���칹��
c����m=12ʱ����Aһ��Ϊ����
d����n=11ʱ����B���ܵķ���ʽ��2��
��2������AΪ�������ҷ���������̼ԭ�Ӷ���ͬһ��ֱ���ϣ���A�Ľṹ��ʽΪ______��
��3������AΪ�������ҷ���������̼ԭ��һ�����棬��һ�������£�1mol A������1mol H2�ӳɣ���A��������______��
��4������BΪ����ͬϵ�ȡһ��������B��ȫȼ�պ���������ͨ��������Ũ���ᣬŨ�������������1.26g����ͨ�������ļ�ʯ�ң���ʯ�ҵ���������4.4g������B�ķ���ʽΪ______�����䱽���ϵ�һ�����ֻ��һ�֣�����ϴ���������B��______�֣�
���𰸡���������1��a����A����B���ܻ�Ϊͬϵ���AΪC5H10��BΪC10H20��ϩ����
b����n=mʱ����B�ķ���ʽ��A��ͬ��A��B��Ϊͬ���칹�壻
c������������Hԭ����Cԭ����Ŀ֮�ȴ���2��1ʱ����Ϊ��������m=12ʱ��AΪ���飻
d����n=11ʱ��B�ķ���ʽΪC10H22��
��2����CH��CH��4��ԭ�ӹ��߿�֪��Ҫʹ��A��5��̼ԭ�ӹ��ߣ�����������A�ķ����б�����2��-C��C-����AΪCH��C-C��C-CH3��
��3������A��H2��������ʵ���֮��1��1�ӳɣ���AΪϩ��C5H10������������̼ԭ�Ӳ����ܹ�ƽ�棬��̼ԭ�ӳ�������ṹ����AΪ3-��-1-��ϩ��
��4��n��CO2��= =0.1 mol��n��H2O��=
=0.1 mol��n��H2O��=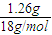 =0.07 mol������B�����ʽΪC5H7��BΪ����ͬϵ���B�ķ���ʽΪCaH2a-6���������ʽ����a��ֵ��ȷ������ʽ�����䱽���ϵ�һ�����ֻ��һ�֣�˵��B���ӵĶԳ��ԽϺã����ݷ���ʽ��д�����������칹�壮
=0.07 mol������B�����ʽΪC5H7��BΪ����ͬϵ���B�ķ���ʽΪCaH2a-6���������ʽ����a��ֵ��ȷ������ʽ�����䱽���ϵ�һ�����ֻ��һ�֣�˵��B���ӵĶԳ��ԽϺã����ݷ���ʽ��д�����������칹�壮
����⣺��1��a����A����B���ܻ�Ϊͬϵ���AΪC5H10��BΪC10H20��ϩ������a��ȷ��
b����n=mʱ����B�ķ���ʽ��A��ͬ��A��B��Ϊͬ���칹�壬��b��ȷ��
c������������Hԭ����Cԭ����Ŀ֮�ȴ���2��1ʱ����Ϊ��������m=12ʱ��AΪ���飬��c��ȷ��
d����n=11ʱ��B�ķ���ʽΪC10H22����d����
�ʴ�Ϊ��d��
��2����CH��CH��4��ԭ�ӹ��߿�֪��Ҫʹ��A��5��̼ԭ�ӹ��ߣ�����������A�ķ����б�����2��-C��C-����AΪCH��C-C��C-CH3��
�ʴ�Ϊ��CH��C-C��C-CH3��
��3������A��H2��������ʵ���֮��1��1�ӳɣ���AΪϩ��C5H10������������̼ԭ�Ӳ����ܹ�ƽ�棬��̼ԭ�ӳ�������ṹ����AΪ3-��-1-��ϩ��
�ʴ�Ϊ��3-��-1-��ϩ��
��4n��CO2��=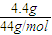 =0.1 mol��n��H2O��=
=0.1 mol��n��H2O��=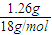 =0.07 mol������B�����ʽΪC5H7��BΪ����ͬϵ���B�ķ���ʽΪCaH2a-6����a����2a-6��=5��7�����a=10����B����ʽΪC10H14���䱽���ϵ�һ�����ֻ��һ�֣���������������У�
=0.07 mol������B�����ʽΪC5H7��BΪ����ͬϵ���B�ķ���ʽΪCaH2a-6����a����2a-6��=5��7�����a=10����B����ʽΪC10H14���䱽���ϵ�һ�����ֻ��һ�֣���������������У�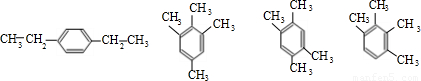 ��
��
�ʴ�Ϊ��C10H14��4��
���������⿼���л����ƶϣ��漰���㡢ͬ���칹�塢�л������������Ŀռ�ṹ�ȣ��Ƕ��л�������֪ʶ���ۺϿ��飬�ܽϺõĿ��鿼����˼ά�������Ѷ��еȣ��Ǹ߿��ȵ����ͣ�
b����n=mʱ����B�ķ���ʽ��A��ͬ��A��B��Ϊͬ���칹�壻
c������������Hԭ����Cԭ����Ŀ֮�ȴ���2��1ʱ����Ϊ��������m=12ʱ��AΪ���飻
d����n=11ʱ��B�ķ���ʽΪC10H22��
��2����CH��CH��4��ԭ�ӹ��߿�֪��Ҫʹ��A��5��̼ԭ�ӹ��ߣ�����������A�ķ����б�����2��-C��C-����AΪCH��C-C��C-CH3��
��3������A��H2��������ʵ���֮��1��1�ӳɣ���AΪϩ��C5H10������������̼ԭ�Ӳ����ܹ�ƽ�棬��̼ԭ�ӳ�������ṹ����AΪ3-��-1-��ϩ��
��4��n��CO2��=
 =0.1 mol��n��H2O��=
=0.1 mol��n��H2O��=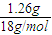 =0.07 mol������B�����ʽΪC5H7��BΪ����ͬϵ���B�ķ���ʽΪCaH2a-6���������ʽ����a��ֵ��ȷ������ʽ�����䱽���ϵ�һ�����ֻ��һ�֣�˵��B���ӵĶԳ��ԽϺã����ݷ���ʽ��д�����������칹�壮
=0.07 mol������B�����ʽΪC5H7��BΪ����ͬϵ���B�ķ���ʽΪCaH2a-6���������ʽ����a��ֵ��ȷ������ʽ�����䱽���ϵ�һ�����ֻ��һ�֣�˵��B���ӵĶԳ��ԽϺã����ݷ���ʽ��д�����������칹�壮����⣺��1��a����A����B���ܻ�Ϊͬϵ���AΪC5H10��BΪC10H20��ϩ������a��ȷ��
b����n=mʱ����B�ķ���ʽ��A��ͬ��A��B��Ϊͬ���칹�壬��b��ȷ��
c������������Hԭ����Cԭ����Ŀ֮�ȴ���2��1ʱ����Ϊ��������m=12ʱ��AΪ���飬��c��ȷ��
d����n=11ʱ��B�ķ���ʽΪC10H22����d����
�ʴ�Ϊ��d��
��2����CH��CH��4��ԭ�ӹ��߿�֪��Ҫʹ��A��5��̼ԭ�ӹ��ߣ�����������A�ķ����б�����2��-C��C-����AΪCH��C-C��C-CH3��
�ʴ�Ϊ��CH��C-C��C-CH3��
��3������A��H2��������ʵ���֮��1��1�ӳɣ���AΪϩ��C5H10������������̼ԭ�Ӳ����ܹ�ƽ�棬��̼ԭ�ӳ�������ṹ����AΪ3-��-1-��ϩ��
�ʴ�Ϊ��3-��-1-��ϩ��
��4n��CO2��=
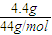 =0.1 mol��n��H2O��=
=0.1 mol��n��H2O��=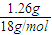 =0.07 mol������B�����ʽΪC5H7��BΪ����ͬϵ���B�ķ���ʽΪCaH2a-6����a����2a-6��=5��7�����a=10����B����ʽΪC10H14���䱽���ϵ�һ�����ֻ��һ�֣���������������У�
=0.07 mol������B�����ʽΪC5H7��BΪ����ͬϵ���B�ķ���ʽΪCaH2a-6����a����2a-6��=5��7�����a=10����B����ʽΪC10H14���䱽���ϵ�һ�����ֻ��һ�֣���������������У� ��
���ʴ�Ϊ��C10H14��4��
���������⿼���л����ƶϣ��漰���㡢ͬ���칹�塢�л������������Ŀռ�ṹ�ȣ��Ƕ��л�������֪ʶ���ۺϿ��飬�ܽϺõĿ��鿼����˼ά�������Ѷ��еȣ��Ǹ߿��ȵ����ͣ�

��ϰ��ϵ�д�
�����Ŀ