��Ŀ����
��Ԫ�����ڱ��д�������λ�õ�Ԫ���ڽṹ�����������������Ƶĵط����ڶ����ڵ�̼�������������������γ��⻯���Ԫ�ص��⻯���H![]() O�⣬����H
O�⣬����H![]() O
O![]() ��̼Ԫ�ص��⻯���CH
��̼Ԫ�ص��⻯���CH![]() �⣬����C
�⣬����C![]() H
H![]() �ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N
�ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N![]() H
H![]() �ȡ�
�ȡ�
��1��̼ԭ��֮����Խ�ϳ���״�ṹ����ԭ��֮��Ҳ�����γ���״�ṹ�����赪ԭ�Ӽ�ֻ�Ե���������ʽ���ӳ���״�����γ��⻯����ϵ���⻯���ͨʽΪ ��
��2����ϵ���е�N![]() H
H![]() �ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N
�ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N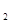 H
H![]() �ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��3����ϵ�����е�NH![]() ��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
![]()
![]()
![]()
I����һ���¶��£���1.5molN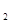 ��6 molH
��6 molH![]() ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
��ʱ��Ӧ�ų�������Ϊ kJ.
H![]() ��ת����= ��
��ת����= ��
���¶��ºϳɰ���Ӧ��ƽ�ⳣ��![]() = ��ֻ�����ֱ���ʽ��
= ��ֻ�����ֱ���ʽ��
II���ڱ����¶Ȳ��䣬��ͬ������ܱ������У�����ʼ�����ʵ�����ΪamolN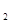 ��bmolH
��bmolH![]() ��cmolNH
��cmolNH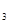 ,ƽ��ʱNH
,ƽ��ʱNH![]() �����ʵ�������Ϊ25%����
�����ʵ�������Ϊ25%����
�ﵽƽ��ʱ��I��II�ų������� ������ĸ���ţ�
A��һ�����
B��ǰ��һ��С�ں���
C��ǰ�ߵ��ڻ�С�ں���
D��ǰ�ߵ��ڻ���ں���
I��II�ϳɰ���ƽ�ⳣ���ֱ�Ϊ![]() ��
��![]() ��ͬ
��ͬ![]()
![]() �����������������=����
�����������������=����
��ʹ��ʼʱ��Ӧ������У�a��ȡֵ��ΧΪ ��
����:��

 �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�
 �ϵ�����Ԫ�������� �ƣ����Ϊ�Խ��߹��ݴ���ش�
�ϵ�����Ԫ�������� �ƣ����Ϊ�Խ��߹��ݴ���ش�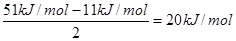 ��
��