��Ŀ����
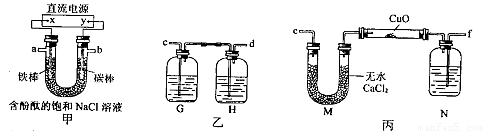
ij��ѧ��ȤС���������ͼ��ʾװ�õ�ⱥ���Ȼ�����Һ�Ʊ�H2��ͨ��H2��ԭ����ͭ�ⶨCu�����ԭ������Ar��Cu����ͬʱ����Cl2�������ԣ�ͼ�мгֺͼ�����������ȥ����
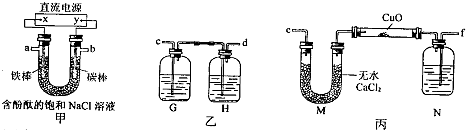
��1��ֱ����Դ�е�X��Ϊ �����������������������������������д����װ��U�ι��з�Ӧ�����ӷ���ʽ�� ��ʵ�鿪ʼ�����������缫��һ���ʵ�������� ��
��2��Ϊ�������ʵ�飬��ȷ������˳��Ϊ��a�� ��b�� ����д���ӵ���ĸ����
��3��װ�����е�Gƿ����Һ����Ϊ ������ĸ����
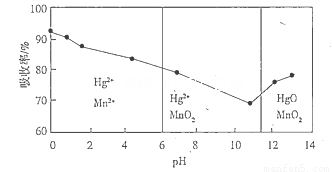
A������KI��ҺB��NaOH��ҺC��Na2S��ҺD��Na2SO3��Һ
Hƿ�ڵķ�Ӧ�����ӷ���ʽΪ�� ��
��4���ڶ�Ӳ�ʲ����Թ��������ͭ��ĩ����ǰ��Ҫ���еIJ���Ϊ�� ��
��5��װ�ñ���Nƿ��ʢ�ŵ��Լ�Ϊ �������� ��
��6��Ϊ�˲ⶨCu�����ԭ��������ijͬѧͨ��ʵ������һ�����ݣ�
I������ͭ��Ʒ����Ϊm1g
��Ӧ��Ӳ�ʲ�������ʣ���������Ϊm2g
III����Ӧǰ��U�ιܼ������������Ϊm3g
������Ӧǰ��ƿ����Һ��������Ϊm4g
����ѡ�������������С��һ�����ݼ���Ar��Cu����Ar��Cu��= ��
�����ѡ���������ݽ��м��㣬�ᵼ��Ar��Cu�� ���ƫ����ƫС������Ӱ�족���������� ��

��1��ֱ����Դ�е�X��Ϊ
��2��Ϊ�������ʵ�飬��ȷ������˳��Ϊ��a��
��3��װ�����е�Gƿ����Һ����Ϊ
A������KI��ҺB��NaOH��ҺC��Na2S��ҺD��Na2SO3��Һ
Hƿ�ڵķ�Ӧ�����ӷ���ʽΪ��
��4���ڶ�Ӳ�ʲ����Թ��������ͭ��ĩ����ǰ��Ҫ���еIJ���Ϊ��
��5��װ�ñ���Nƿ��ʢ�ŵ��Լ�Ϊ
��6��Ϊ�˲ⶨCu�����ԭ��������ijͬѧͨ��ʵ������һ�����ݣ�
I������ͭ��Ʒ����Ϊm1g
��Ӧ��Ӳ�ʲ�������ʣ���������Ϊm2g
III����Ӧǰ��U�ιܼ������������Ϊm3g
������Ӧǰ��ƿ����Һ��������Ϊm4g
����ѡ�������������С��һ�����ݼ���Ar��Cu����Ar��Cu��=
�����ѡ���������ݽ��м��㣬�ᵼ��Ar��Cu��
��������1�����ݵ��ԭ��������������������ӵ�Դ�ĸ��������е�ⱥ��ʳ��ˮ��
��2����ͨ��H2��ԭ����ͭ�ⶨCu�����ԭ������Ar��Cu������a��f����ͬʱ����Cl2�������ԡ�����b��c��
��3��A��C��D�е�I-��S2-��SO32-���л�ԭ�ԣ�����Cl2��SO32-��Ӧû�����������ж�������ֱ���ŷŵ������У����������ü�������������������װ����Hƿ�����ӷ���ʽ��д����
��4�������ǿ�ȼ�����壬��������ϣ�������������ըΣ�գ����Լ�������֮ͭǰҪͨ�������ž�װ���ڵĿ����������H2�Ĵ��ȷ�ֹ��ը����
��5����ⱥ���Ȼ�����Һ�Ʊ��������к���ˮ������������ʵ��ǰҪ��ȥˮ��������ѡŨ�������H2����ֹӲ���Թ�ը�ѣ�
��6����CuO+H2=Cu+H2O��m=80-64=16������������Ϊm1-m2��������Ӧ��Ӳ�ʲ�������ʣ���������Ϊm2g������Cu������Ϊm2g�����ݻ�ѧ����ʽ�Ϳ����Ar��Cu����
�ڿ����е�ˮͨ��e���ܽ���U�����m3�������յ���Ar��Cu��ƫС��
��2����ͨ��H2��ԭ����ͭ�ⶨCu�����ԭ������Ar��Cu������a��f����ͬʱ����Cl2�������ԡ�����b��c��
��3��A��C��D�е�I-��S2-��SO32-���л�ԭ�ԣ�����Cl2��SO32-��Ӧû�����������ж�������ֱ���ŷŵ������У����������ü�������������������װ����Hƿ�����ӷ���ʽ��д����
��4�������ǿ�ȼ�����壬��������ϣ�������������ըΣ�գ����Լ�������֮ͭǰҪͨ�������ž�װ���ڵĿ����������H2�Ĵ��ȷ�ֹ��ը����
��5����ⱥ���Ȼ�����Һ�Ʊ��������к���ˮ������������ʵ��ǰҪ��ȥˮ��������ѡŨ�������H2����ֹӲ���Թ�ը�ѣ�
��6����CuO+H2=Cu+H2O��m=80-64=16������������Ϊm1-m2��������Ӧ��Ӳ�ʲ�������ʣ���������Ϊm2g������Cu������Ϊm2g�����ݻ�ѧ����ʽ�Ϳ����Ar��Cu����
�ڿ����е�ˮͨ��e���ܽ���U�����m3�������յ���Ar��Cu��ƫС��
����⣺��1�����ݵ��ԭ��������������������ӵ�Դ�ĸ�������X��ӦΪ���������е�ⱥ��ʳ��ˮ����Ӧ�����ӷ���ʽΪ2Cl-+2H2O2OH-+H2��+Cl2����
�ʴ�Ϊ��������2Cl-+2H2O
2OH-+H2��+Cl2�� �������ݳ�����Һ����ɫ��ɺ�ɫ��������Χ��ɢ��
��2����ͨ��H2��ԭ����ͭ�ⶨCu�����ԭ������Ar��Cu������a��f����ͬʱ����Cl2�������ԡ�����b��c���ʴ�Ϊ��f��c��
��3��A��C��D�е�I-��S2-��SO32-���л�ԭ�ԣ�����Cl2��SO32-��Ӧû������ѡA��C�������ж�������ֱ���ŷŵ������У����������ü�������������������װ����Hƿ��Һ������������ն������������ֹ������Ⱦ�����ӷ���ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O���ʴ�Ϊ��A��C��Cl2+2OH-=Cl-+ClO-+H2O��
��4�������ǿ�ȼ�����壬��������ϣ�������������ըΣ�գ����Լ�������֮ͭǰҪͨ�������ž�װ���ڵĿ����������H2�Ĵ��ȷ�ֹ��ը�����ʴ�Ϊ�����������Ĵ��ȷ�ֹ��ը��
��5��ͨ��H2��ԭ����ͭ�ⶨCu�����ԭ������Ar��Cu���������ڵ�ⱥ���Ȼ�����Һ�Ʊ��������к���ˮ������������ʵ��ǰҪ��ȥˮ��������ѡŨ���ᣬ����H2����ֹӲ���Թ�ը�ѣ��ʴ�Ϊ��Ũ�������H2����ֹӲ���Թ�ը�ѣ�
��6������CuO��Cu֪��m=80-64=16������������Ϊm1-m2��������Ӧ��Ӳ�ʲ�������ʣ���������Ϊm2g������Cu������Ϊm2g�����ݻ�ѧ����ʽ��
CuO+H2=Cu+H2O��m Ar��Cu�� 16
m2 m1-m2
���Ar��Cu��=
��
��ƫС�������е�ˮͨ��e���ܽ���U�����m3�������յ���Ar��Cu��ƫС��
�ʴ�Ϊ����
����ƫС�������е�ˮͨ��e���ܽ���U�����m3�������յ���Ar��Cu��ƫС��
�ʴ�Ϊ��������2Cl-+2H2O
| ||
��2����ͨ��H2��ԭ����ͭ�ⶨCu�����ԭ������Ar��Cu������a��f����ͬʱ����Cl2�������ԡ�����b��c���ʴ�Ϊ��f��c��
��3��A��C��D�е�I-��S2-��SO32-���л�ԭ�ԣ�����Cl2��SO32-��Ӧû������ѡA��C�������ж�������ֱ���ŷŵ������У����������ü�������������������װ����Hƿ��Һ������������ն������������ֹ������Ⱦ�����ӷ���ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O���ʴ�Ϊ��A��C��Cl2+2OH-=Cl-+ClO-+H2O��
��4�������ǿ�ȼ�����壬��������ϣ�������������ըΣ�գ����Լ�������֮ͭǰҪͨ�������ž�װ���ڵĿ����������H2�Ĵ��ȷ�ֹ��ը�����ʴ�Ϊ�����������Ĵ��ȷ�ֹ��ը��
��5��ͨ��H2��ԭ����ͭ�ⶨCu�����ԭ������Ar��Cu���������ڵ�ⱥ���Ȼ�����Һ�Ʊ��������к���ˮ������������ʵ��ǰҪ��ȥˮ��������ѡŨ���ᣬ����H2����ֹӲ���Թ�ը�ѣ��ʴ�Ϊ��Ũ�������H2����ֹӲ���Թ�ը�ѣ�
��6������CuO��Cu֪��m=80-64=16������������Ϊm1-m2��������Ӧ��Ӳ�ʲ�������ʣ���������Ϊm2g������Cu������Ϊm2g�����ݻ�ѧ����ʽ��
CuO+H2=Cu+H2O��m Ar��Cu�� 16
m2 m1-m2
���Ar��Cu��=
| 16m2 |
| (m1-m2) |
��ƫС�������е�ˮͨ��e���ܽ���U�����m3�������յ���Ar��Cu��ƫС��
�ʴ�Ϊ����
| 16m2 |
| (m1-m2) |
������������ԭ��Ӧ�á��������顢������ԭ����ͭʵ�������Ԫ�����ԭ�������IJⶨ��ʵ����Ƶȣ�

��ϰ��ϵ�д�
�����Ŀ
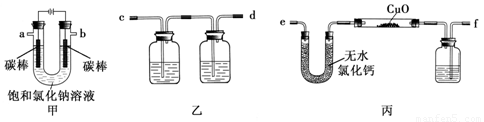
��15�֣�ij��ѧ��ȤС���������ͼװ�ü�ⱥ���Ȼ�����Һ�����õ�������H2��ԭCuO��ĩ���ⶨCu�����ԭ������Ar(Cu)��ͬʱ���������������ԣ�ͼ�мгֺͼ�����������ȥ��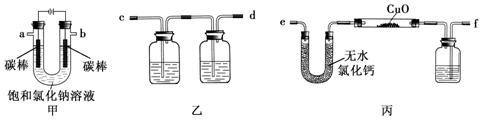
��1��д��װ�ü��з�Ӧ�����ӷ���ʽ�� ��
��2��Ϊ�������ʵ�飬��ȷ������˳��Ϊa�� ��b�� ����д���ӵ���ĸ����
��3��װ�����е�һ�����ƿ�ڵ���Һ�����ǣ� ��
| A�����۵⻯����Һ | B��NaOH��Һ |
| C��FeCl2��KSCN�����Һ | D��Na2SO3��Һ |
 ��Ӳ�ʲ������������ͭ��ĩ����ǰ������еIJ���Ϊ ��
��Ӳ�ʲ������������ͭ��ĩ����ǰ������еIJ���Ϊ ����5��װ�ñ��й��ƿ��ʢ�ŵ��Լ�Ϊ �������� ��
��6��Ϊ�˲ⶨCu�����ԭ��������ijͬѧͨ��ʵ���ñ�װ�÷�Ӧǰ���������ݣ���Ʒ����Ϊm1g����Ӧ��Ӳ�ʲ�������ʣ���������Ϊm2g����Ӧǰ��U�ܼ����й���������Ϊm3g����Ӧǰ��ϴ��ƿ������Һ��������Ϊm4g��
����ѡ�������������С��һ�����ݼ���Ar(Cu)��Ar(Cu)= ��
�����ѡ�����������ݽ��м��㣬�ᵼ��Ar(Cu) ���ƫ����ƫС������Ӱ�족���������� ��