��Ŀ����
7��ʵ��������NaOH��������1.0mol•L-1��NaOH��Һ500mL�����������������ձ���100mL��Ͳ��500mL����ƿ�ܲ�������������ƽ�������룩��ҩ����1����ȱ�ٵ������ǽ�ͷ�ι�
��2��ʵ�������õ��������������÷ֱ��ǣ����衢�����ܽ⣻����
��3�������ƹ����У��ܽ⡢ת����Һ��δ���ձ��Ͳ���������ϴ�ӣ����������õ���ҺŨ�ȵ�Ӱ����ƫС���ƫ����ƫС������Ӱ�족����
��4������ȷ���������Һ����ȡ��10mL�������ʵ���Ũ��Ϊ1.0 mol•L-1����NaOH0.4g��������10mL��Һ��ˮϡ�͵�100mL��������Һ��NaOH�����ʵ���Ũ��Ϊ0.1 mol•L-1��
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2������ʹ�ò������IJ�������������;��
��3���ܽ⡢ת����Һ��δ���ձ��Ͳ���������ϴ�ӣ��ᵼ���ʵ���ʧ��
��4����ҺΪ��һ�ȶ���ɢϵ��ȡ�������������ԭ��ҺŨ����ȣ�����m=CVM�����㣻
������Һϡ��ǰ�����ʵ����ʵ����������ϡ�ͺ����ʵ����ʵ���Ũ�ȣ�
��� �⣺��1���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪�����������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ�
�ʻ�ȱ�ٵ������ǽ�ͷ�ιܣ��ʴ�Ϊ����ͷ�ιܣ�
��2�������ƹ����У��ܽ��������ƹ���ʱ����������;�ǣ����裬�����ܽ⣻����Һʱ����������;��������
�ʴ�Ϊ�����衢�����ܽ⣻������
��3���ܽ⡢ת����Һ��δ���ձ��Ͳ���������ϴ�ӣ��ᵼ���ʵ���ʧ������ҺŨ��ƫС���ʴ�Ϊ��ƫС��
��4����ҺΪ��һ�ȶ���ɢϵ��ȡ�������������ԭ��ҺŨ����ȣ�����ȡ��10mL�������ʵ���Ũ��Ϊ1.0mol/L��
����m=CVM��֪�����е��������Ƶ�����m=1.0mol/L��0.01L��40g/mol=0.4g��
��Һϡ��ǰ�����ʵ����ʵ������䣬��10mL��Һ��ˮϡ�͵�100mL��������Һ�����ʵ����ʵ���Ũ��Ϊ$\frac{0.01L��1.0mol/L}{0.1L}$=0.1mol/L��
�ʴ�Ϊ��1��0.4��0.1��
���� ���⿼�����ʵ���Ũ�ȼ��㣬��Ŀ�ѶȲ���ע���йؼ��㹫ʽ�����ã�������Һ���ص��Լ���Һϡ��ǰ�����ʵ����ʵ��������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ˮ���й̶����۵㣬����ͨ������û�� | |
| B�� | ˮ�ࡢ�������մ����ڴ�ͳ�����ǽ������� | |
| C�� | ����ֺ���ͨ�ֵ����Ԫ����ȫ��ͬ������ʴ���ܲ�ͬ | |
| D�� | �Ȼ�狀͵��ʵ��ڼ���ʱ�������������ʲ�ͬ |
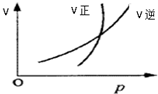
| A�� | N2O4��g��?2NO2��g�� | B�� | CO2��g��+C��s��?2CO��g�� | ||
| C�� | 2SO2��g��+O2��g��?2SO3��g�� | D�� | 4NH3��g��+5O2��g��?4NO��g��+6H2O��g�� |
| A�� | ��������ˮ�� | B�� | ������������ʪ�� | ||
| C�� | 84����Һ�������� | D�� | ������ʳƷ���ڵ������� |
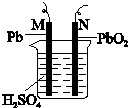 ��֪Ǧ���صķ�Ӧԭ���ǣ�Pb��s��+PbO2��s��+2H2SO4��aq���T2PbSO4��s��+2H2O��l����H��0ͼΪǦ���ص�ʾ��ͼ������˵����ȷ���ǣ�������
��֪Ǧ���صķ�Ӧԭ���ǣ�Pb��s��+PbO2��s��+2H2SO4��aq���T2PbSO4��s��+2H2O��l����H��0ͼΪǦ���ص�ʾ��ͼ������˵����ȷ���ǣ�������| A�� | ���ʱ�������ĵ缫��ӦʽΪ��PbSO4+2e-�TPb+SO42- | |
| B�� | ���ʱ����N���ӵ�Դ��������ü�����PbO2 | |
| C�� | �ŵ�ʱ��c��H2SO4�����䣬�������������� | |
| D�� | �ŵ�ʱ��NΪ��������缫��ӦʽΪ��PbO2+SO42-+4H++2e-�TPbSO4+2H2O |
��1��Ϊ�����ľ���е����ӣ�ȡ������������ˮ��������Һ�ֳ��ķݣ��������ʵ�鱨�棨�ں�������д��ص����ݣ���
| ʵ�鲽�� | ʵ������ | ʵ����� |
��ȡ��һ����Һ������ϡ�����������װ�õ��Թ��У��ѳ����ʯ��ˮ�����ձ���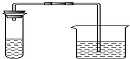 | ���ݲ������� ���ʯ��ˮ����� | ֤������̼������� |
| ��ȡ�ڶ�����Һ���ֱ�μ�ϡ������Ȼ��� | ��ɫ���� | ֤��������������� |
| ��ȡ��������Һ�������BaCl2��Һ�����ˣ���ȥSO42-��CO32-���ٵμ�AgNO3��Һ��ϡ���� | �а�ɫ������������ | ֤������������ |
| ����ȡ���ķ���Һ������ɫ��Ӧ | ����ɫ�ܲ��� �������ɫ | ֤������K+ |
��3��д��������й�����Ļ�ѧ����ʽK2SO4+BaCl2=BaSO4��+2KCl��
| A�� | pH=2��pH=1��������c��H+��֮��Ϊ1��10 | |
| B�� | 0.2mol/L��0.1mol/L������c��H+��֮��Ϊ2��1 | |
| C�� | pH���NaOH��Ba��OH��2����Һ��c��OH-��֮��Ϊ1��2 | |
| D�� | 0.1mol/L������0.1mol/L��������Һ��c��CH3COO-��֮��Ϊ1��1 |
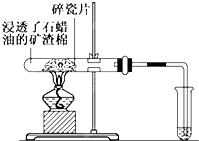 ʯ���ͣ���Ҫ�Ǻ�17��̼ԭ������Һ̬���������ֽ�ʵ�鰴��ͼ�����У�
ʯ���ͣ���Ҫ�Ǻ�17��̼ԭ������Һ̬���������ֽ�ʵ�鰴��ͼ�����У�