��Ŀ����
��2013?����һģ��I����һ���ݻ�Ϊ2L���ܱ������м���2molN2��6molH2���������·�Ӧ��
N2��g��+3H2��g��?2NH3��g����H��0��5min��ﵽƽ�⣬���c��NH3��=0.5mol/L��
��1���������´˷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽK=
���¶����ߣ���Kֵ
��2���ӷ�Ӧ��ʼ��ƽ�⣬��H2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ��
��3����ƽ��ʱ������1mol N2��3mol H2������ͬ�¶����ٴ�ƽ��ʱc��NH3��
��25�棬��0.4mol/LCH3COOH��Һ��0.2mol/LNaOH��Һ��100mL��Ϻ�pH=5�������Ϻ���Һ�����Ϊ����Һ���֮�ͣ�
��1�������Һ������Ũ���ɴ�С��˳���ǣ�
��2����c��CH3COO-��+c��CH3COOH��=
��c��CH3COO-��-c��CH3COOH��=
����֪25��ʱ��Ksp[Fe��OH��3]=8��10-39�����¶��·�ӦFe��OH��3+3H+?Fe3++3H2O��ƽ�ⳣ��Ϊ
N2��g��+3H2��g��?2NH3��g����H��0��5min��ﵽƽ�⣬���c��NH3��=0.5mol/L��
��1���������´˷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽK=
| c2(NH3) |
| c(N2)��c3(H2) |
| c2(NH3) |
| c(N2)��c3(H2) |
��С
��С
������������С�����䡱����2���ӷ�Ӧ��ʼ��ƽ�⣬��H2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ��
0.15mol/��L?min��
0.15mol/��L?min��
����3����ƽ��ʱ������1mol N2��3mol H2������ͬ�¶����ٴ�ƽ��ʱc��NH3��
��
��
0.25mol/L�������������������=������25�棬��0.4mol/LCH3COOH��Һ��0.2mol/LNaOH��Һ��100mL��Ϻ�pH=5�������Ϻ���Һ�����Ϊ����Һ���֮�ͣ�
��1�������Һ������Ũ���ɴ�С��˳���ǣ�
c��CH3COO-����c��Na+����c��H+����c��OH-��
c��CH3COO-����c��Na+����c��H+����c��OH-��
����2����c��CH3COO-��+c��CH3COOH��=
0.2
0.2
moL/L��c��CH3COO-��-c��CH3COOH��=
2����10-5-10-9��
2����10-5-10-9��
mol/L����֪25��ʱ��Ksp[Fe��OH��3]=8��10-39�����¶��·�ӦFe��OH��3+3H+?Fe3++3H2O��ƽ�ⳣ��Ϊ
8��103
8��103
������ʽ�����㣩 ��0.001mob/L FeCl3��Һ��ͨ�백��������仯���Բ��ƣ�����ʼ����ʱ��Һ��pHΪ2.3
2.3
����lg5=0.7����������1����ѧƽ�ⳣ������ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ���ݴ���д��
�÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ����ݴ��ж��¶ȶ�ƽ�ⳣ����Ӱ�죻
��2������v=
����v��NH3�����ٸ�������֮�ȵ��ڻ�ѧ������֮�ȼ���v��H2����
��3����ƽ��ʱ������1mol N2��3mol H2����ЧΪԭƽ���������һ��������ѹǿ����ƽ�ⲻ�ƶ�����c��NH3��=0.25mol/L����ƽ�����淴Ӧ�ƶ���
��1��0.4mol/LCH3COOH��Һ��0.2mol/LNaOH��Һ��100mL��ϣ���Ӧ�����Һ�к�CH3COOH��CH3COONa��0.02mol����ҺpH=5��˵������ĵ���̶ȴ��ڴ������ˮ��̶ȣ��ݴ��ж�����
��2���ٸ��������غ���㣻
�ڸ��������غ㡢����غ���㣻
����Ksp[Fe��OH��3]=c��Fe3+��?c3��OH-����Kw=c��H+��?c��OH-����Fe��OH��3+3H+?Fe3++3H2O��ƽ�ⳣ������ʽ���ɵ�3�������Ĺ�ϵ�����м��㣮
����Ksp[Fe��OH��3]=c��Fe3+��?c3��OH-������c��OH-�����ٸ���Kw=c��H+��?c��OH-������c��H+����������pH=-lgc��H+�����㣮
�÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ����ݴ��ж��¶ȶ�ƽ�ⳣ����Ӱ�죻
��2������v=
| ��c |
| ��t |
��3����ƽ��ʱ������1mol N2��3mol H2����ЧΪԭƽ���������һ��������ѹǿ����ƽ�ⲻ�ƶ�����c��NH3��=0.25mol/L����ƽ�����淴Ӧ�ƶ���
��1��0.4mol/LCH3COOH��Һ��0.2mol/LNaOH��Һ��100mL��ϣ���Ӧ�����Һ�к�CH3COOH��CH3COONa��0.02mol����ҺpH=5��˵������ĵ���̶ȴ��ڴ������ˮ��̶ȣ��ݴ��ж�����
��2���ٸ��������غ���㣻
�ڸ��������غ㡢����غ���㣻
����Ksp[Fe��OH��3]=c��Fe3+��?c3��OH-����Kw=c��H+��?c��OH-����Fe��OH��3+3H+?Fe3++3H2O��ƽ�ⳣ������ʽ���ɵ�3�������Ĺ�ϵ�����м��㣮
����Ksp[Fe��OH��3]=c��Fe3+��?c3��OH-������c��OH-�����ٸ���Kw=c��H+��?c��OH-������c��H+����������pH=-lgc��H+�����㣮
����⣺��1��N2��g��+3H2��g��?2NH3��g��ƽ�ⳣ��k=
���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ���ƽ�ⳣ����С��
�ʴ�Ϊ��
������
��2��5min��ﵽƽ�⣬���c��NH3��=0.5mol/L����v��NH3��=
=0.1mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ�v��H2��=1.5v��NH3��=1.5��0.1mol/��L?min��=0.15mol/��L?min����
�ʴ�Ϊ��0.15mol/��L?min����
��3����ƽ��ʱ������1mol N2��3mol H2����ЧΪԭƽ���������һ��������ѹǿ����ƽ�ⲻ�ƶ�����c��NH3��=0.25mol/L����ƽ�����淴Ӧ�ƶ�����ƽ��ʱc��NH3����0.25mol/L��
�ʴ�Ϊ������
��1��0.4mol/LCH3COOH��Һ��0.2mol/LNaOH��Һ��100mL��ϣ���Ӧ�����Һ�к�CH3COOH��CH3COONa��0.02mol����ҺpH=5����Һ�����ԣ���c��H+����c��OH-����˵������ĵ���̶ȴ��ڴ������ˮ��̶ȣ���c��CH3COO-����c��Na+����������������ʣ�������ȫ���룬��c��Na+����c��H+����������Ũ���ɴ�С��˳���ǣ�c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
��2���ٷ�Ӧ�����Һ�к�CH3COOH��CH3COONa��0.02mol����Һ���Ϊ200mL�����������غ��֪��c��CH3COO-��+c��CH3COOH��=
=0.2moL/L��
�ʴ�Ϊ��0.2��
�ڸ��������غ��У�c��CH3COO-��+c��CH3COOH��=2c��Na+����
���ݵ���غ��У�c��Na+��+c��H+��=c��OH-��+c��CH3COO-����
�����ã�c��CH3COO-��+c��CH3COOH��=2[c��OH-��+c��CH3COO-��-c��H+��]
��c��CH3COO-��-c��CH3COOH��=2[c��H+��-c��OH-��]=2����10-5-10-9��mol/L
�ʴ�Ϊ��2����10-5-10-9����
8��Fe��OH��3+3H+?Fe3++3H2O��ƽ�ⳣ������ʽk=
����Ksp[Fe��OH��3]=c��Fe3+��?c3��OH-����Kw=c��H+��?c��OH-������֪ʽk=
=
=
=8��103��
��0.001mob/L FeCl3��Һ��ͨ�백��������仯���Բ��ƣ�����ʼ����ʱ��Һ��c��OH-��=
=2��10-12mol/L����c��H+��=
=5��10-3mol/L����pH=-lgc��H+��=-lg5��10-3=2.3��
�ʴ�Ϊ��8��103��2.3��
| c2(NH3) |
| c(N2)��c3(H2) |
�ʴ�Ϊ��
| c2(NH3) |
| c(N2)��c3(H2) |
��2��5min��ﵽƽ�⣬���c��NH3��=0.5mol/L����v��NH3��=
| 0.5mol/L |
| 5min |
�ʴ�Ϊ��0.15mol/��L?min����
��3����ƽ��ʱ������1mol N2��3mol H2����ЧΪԭƽ���������һ��������ѹǿ����ƽ�ⲻ�ƶ�����c��NH3��=0.25mol/L����ƽ�����淴Ӧ�ƶ�����ƽ��ʱc��NH3����0.25mol/L��
�ʴ�Ϊ������
��1��0.4mol/LCH3COOH��Һ��0.2mol/LNaOH��Һ��100mL��ϣ���Ӧ�����Һ�к�CH3COOH��CH3COONa��0.02mol����ҺpH=5����Һ�����ԣ���c��H+����c��OH-����˵������ĵ���̶ȴ��ڴ������ˮ��̶ȣ���c��CH3COO-����c��Na+����������������ʣ�������ȫ���룬��c��Na+����c��H+����������Ũ���ɴ�С��˳���ǣ�c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
��2���ٷ�Ӧ�����Һ�к�CH3COOH��CH3COONa��0.02mol����Һ���Ϊ200mL�����������غ��֪��c��CH3COO-��+c��CH3COOH��=
| 0.02mol+0.02mol |
| 0.2L |
�ʴ�Ϊ��0.2��
�ڸ��������غ��У�c��CH3COO-��+c��CH3COOH��=2c��Na+����
���ݵ���غ��У�c��Na+��+c��H+��=c��OH-��+c��CH3COO-����
�����ã�c��CH3COO-��+c��CH3COOH��=2[c��OH-��+c��CH3COO-��-c��H+��]
��c��CH3COO-��-c��CH3COOH��=2[c��H+��-c��OH-��]=2����10-5-10-9��mol/L
�ʴ�Ϊ��2����10-5-10-9����
8��Fe��OH��3+3H+?Fe3++3H2O��ƽ�ⳣ������ʽk=
| c(Fe3+) |
| c3(H+) |
| c(Fe3+) |
| c3(H+) |
| Ksp |
| Kw3 |
| 8��10-39 |
| (1��10-14)3 |
��0.001mob/L FeCl3��Һ��ͨ�백��������仯���Բ��ƣ�����ʼ����ʱ��Һ��c��OH-��=
| 3 |
| ||
| 10-14 |
| 2��10-12 |
�ʴ�Ϊ��8��103��2.3��
��������������ƴ������Ŀ���漰��ѧƽ���뷴Ӧ���ʡ�����ˮ�⡢ƽ�ⳣ�����йؼ���ȣ���Ŀ�ۺ��Խϴ��Ѷ��еȣ���һ�����������������⣬ע�����֪ʶ���������գ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
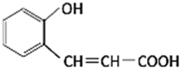 ��2013?����һģ����ͼ�Ǻϳ������㶹�ع��̵��м������ڸ����ʵ�˵������ȷ���ǣ�������
��2013?����һģ����ͼ�Ǻϳ������㶹�ع��̵��м������ڸ����ʵ�˵������ȷ���ǣ�������