��Ŀ����
Ϊʵ��ʵ��Ŀ�ģ�ѡ�õ�װ�á�ʵ���������ȷ���ǣ� ��
ʵ��Ŀ�� | ʵ�鲽���װ�� | |
A | ̽���¶ȶԷ�Ӧ���ʵ�Ӱ�� | ��ͬ�¶��£�ȡ0.1 mol/LKI ��Һ���������ȼ��������Һ���ټ���0.1 mol/L ���ᣬ��¼��Һ������ɫ��ʱ�� |
B | ����100mL1.0mol/L NaOH��Һ | ��100mL����ƿ�м���4. 0gNaOH���壬��ˮ���̶��� |
C | ��֤�������������������� |
�����缫�����μ����軯����Һ |
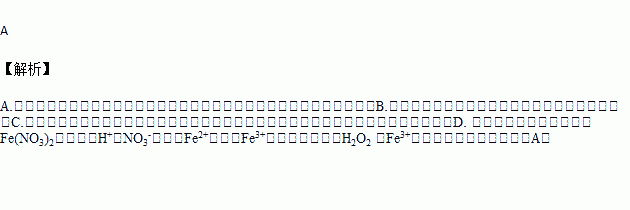
D | �Ƚ�H2O2 ��Fe3+�������� | �������ữ��˫��ˮ����Fe(NO3)2��Һ�� |
A. A B. B C. C D. D
�ҹ��ӹ������ijԭ�Ͼ��ⶨ��Ҫ����A��B��C��D��E����ǰ������Ԫ�أ���ԭ��������������Ԫ��A��B��C��D��E��ԭ�ӽṹ����Ϣ���£�
Ԫ�� | Ԫ�����ʻ�ԭ�ӽṹ |
A | ���ڱ���ԭ�Ӱ뾶��С��Ԫ�� |
B | ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ |
C | �����p�������� |
D | λ�ڶ����ڣ���ԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������3�� |
E | λ�ڵ������ڣ��ڲ����ȫ��������ԭ�ӵ�������������A����ͬ |
��ش���������(��A��B��C��D��E����Ӧ��Ԫ�ط�������)��
��1��B��C��D��һ��������С����Ϊ_________________��
��2��E�Ļ�̬ԭ�ӵĺ�������Ų�ʽΪ____________��
��3��A2B2D4��������������÷�����B���ӻ���ʽΪ______________��1 mol A2B2D4�����к��ЦҼ���ĿΪ_____________��
��4���뻯����BD��Ϊ�ȵ�����������ӻ�ѧʽΪ_____________(��дһ��)��
��5��B2A6��C2A4�����о�����18�����ӣ����ǵķе����ϴ���Ҫԭ����____________��
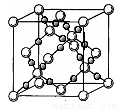
��6��BD2�ڸ��¸�ѹ�����γɾ���ľ�����ͼ��ʾ��һ���þ����к�___________��Dԭ�ӡ�



 ��2OH��= CaCO3����CO
��2OH��= CaCO3����CO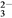 ��2H2O
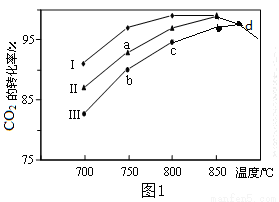
��2H2O CH3OH��H2O����������ͬ���ü״��ϳɷ�Ӧ�ڲ�ͬ�������������·�Ӧ��ͬʱ���CO2��ת�����淴Ӧ�¶ȵı仯��ͼ����ʾ��
CH3OH��H2O����������ͬ���ü״��ϳɷ�Ӧ�ڲ�ͬ�������������·�Ӧ��ͬʱ���CO2��ת�����淴Ӧ�¶ȵı仯��ͼ����ʾ��

 C2H4��g��+4H2O��g����H����ͬ�¶���ƽ��ʱ��������̬���ʵ����ʵ�����ͼ2��ʾ��
C2H4��g��+4H2O��g����H����ͬ�¶���ƽ��ʱ��������̬���ʵ����ʵ�����ͼ2��ʾ��