��Ŀ����
��14�֣���ͼ�ļס�����������������ɱ�ѹǿ���䣬�ұ���������䡣���������зֱ����1 mol A��3 mol B����ʱ�����������Ϊ500 mL���¶�ΪT�档�����¶Ȳ��䣬������ӦA(g)+3B(g) 2C(g)+D(s)����H��0��
2C(g)+D(s)����H��0��
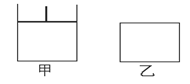
 ��1������ѡ���У��ܱ������������з�Ӧһ���ﵽƽ��״̬����________��
��1������ѡ���У��ܱ������������з�Ӧһ���ﵽƽ��״̬����________��
A��������������������ʱ��ı� B��2 v��(C)��3 v��(B)
C��A��Bת������� D�� ����D����������ʱ��ı�
��2��2 min��������еĻ�ѧ��Ӧ�ﵽƽ�⣬ ���C
���C ��Ũ��Ϊ2 mol/L����ʱ���������Ϊ________mL��B��ת���ʦ���(B)Ϊ________��
��Ũ��Ϊ2 mol/L����ʱ���������Ϊ________mL��B��ת���ʦ���(B)Ϊ________��
��3���������з�Ӧ �ﵽƽ������Ҫ��ʱ��______2 min���������������B��ת���ʦ�
�ﵽƽ������Ҫ��ʱ��______2 min���������������B��ת���ʦ� ��(B) ______����(B) ���������������
��(B) ______����(B) ���������������
��4���������������䣬����������淴Ӧ����ʼ��Ӧ����ƽ�⣬Ҫ��ƽ��ʱC�����ʵ����루2����ƽ��ʱC�����ʵ�����ȣ�����Ҫ����C�����ʵ���n(C)��________mol������D�����ʵ���n (D)Ӧ�����������Ϊ________________________��
 2C(g)+D(s)����H��0��
2C(g)+D(s)����H��0��
 ��1������ѡ���У��ܱ������������з�Ӧһ���ﵽƽ��״̬����________��
��1������ѡ���У��ܱ������������з�Ӧһ���ﵽƽ��״̬����________��A��������������������ʱ��ı� B��2 v��(C)��3 v��(B)
C��A��Bת������� D�� ����D����������ʱ��ı�
��2��2 min��������еĻ�ѧ��Ӧ�ﵽƽ�⣬
 ���C
���C ��Ũ��Ϊ2 mol/L����ʱ���������Ϊ________mL��B��ת���ʦ���(B)Ϊ________��
��Ũ��Ϊ2 mol/L����ʱ���������Ϊ________mL��B��ת���ʦ���(B)Ϊ________����3���������з�Ӧ
 �ﵽƽ������Ҫ��ʱ��______2 min���������������B��ת���ʦ�
�ﵽƽ������Ҫ��ʱ��______2 min���������������B��ת���ʦ� ��(B) ______����(B) ���������������
��(B) ______����(B) �����������������4���������������䣬����������淴Ӧ����ʼ��Ӧ����ƽ�⣬Ҫ��ƽ��ʱC�����ʵ����루2����ƽ��ʱC�����ʵ�����ȣ�����Ҫ����C�����ʵ���n(C)��________mol������D�����ʵ���n (D)Ӧ�����������Ϊ________________________��
��14�֣�
��1��A D
��2��400��40%
��3��������
��4��2��n (D)��0.6 mol
��1��A D
��2��400��40%
��3��������
��4��2��n (D)��0.6 mol
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2Z(g) ��H< 0��Ӧ�仯���������ʵ�Ũ���뷴Ӧ��ʱ��仯��ϵ��ͼ��t2��t3�������߱仯�������������������ĸı��������
2Z(g) ��H< 0��Ӧ�仯���������ʵ�Ũ���뷴Ӧ��ʱ��仯��ϵ��ͼ��t2��t3�������߱仯�������������������ĸı��������
 N2O4 ��H<0�������ƿ����100��ķ�ˮ�У������м��������У�����ɫ ��ƽ�������� ������ ��ѹǿ ���ܶȣ����в���ı����
N2O4 ��H<0�������ƿ����100��ķ�ˮ�У������м��������У�����ɫ ��ƽ�������� ������ ��ѹǿ ���ܶȣ����в���ı���� CO2(g)+H2(g)��һ��ʱ���ﵽƽ�⡣�Ը�ƽ��״̬������ȷ����
CO2(g)+H2(g)��һ��ʱ���ﵽƽ�⡣�Ը�ƽ��״̬������ȷ���� bB(��)�ﵽƽ������¶Ȳ��䣬�������������һ�������ﵽ��ƽ��ʱ��B��Ũ����ԭ����40%���� (�� )
bB(��)�ﵽƽ������¶Ȳ��䣬�������������һ�������ﵽ��ƽ��ʱ��B��Ũ����ԭ����40%���� (�� ) xP(g)+2Q(g)������Ӧ�ﵽƽ��ʱ������4 mol Q�������P��ƽ��Ũ��Ϊ0.4 mol��L��1��������������ȷ����
xP(g)+2Q(g)������Ӧ�ﵽƽ��ʱ������4 mol Q�������P��ƽ��Ũ��Ϊ0.4 mol��L��1��������������ȷ���� 2Z(g)����H��0�����ı�ij���������ﵽ��ƽ�������������ȷ����(����).
2Z(g)����H��0�����ı�ij���������ﵽ��ƽ�������������ȷ����(����). 2C(g)��D(g) �Ѵ�ƽ��״̬���ǣ� ��
2C(g)��D(g) �Ѵ�ƽ��״̬���ǣ� �� CH3OH(g)��
CH3OH(g)��