��Ŀ����
��5�֣�ʹ������ͬ��Ʒ�IJ������������γ����������������ڽ��Ͳ�Ʒ�������ɱ�����ʱҲ������ijЩ��������ۺ����á������dz��Ѿ������ֹ�ϵ�Ĺ��������ܵؽ����������������������ʵ�����н��ͣ�
��1��ʯ��ʯ��ʯ����������������������
��2����ˮ���γ����ȼ������������������Ϣ���ȼ������Ҫ��ѧ��ӦΪ��ⱥ��ʳ��ˮ��2NaCl+2H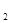 O
O 2NaOH+Cl
2NaOH+Cl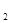
 +H
+H
 ��
��
��1��ʯ��ʯ��ʯ����������������������
��2����ˮ���γ����ȼ������������������Ϣ���ȼ������Ҫ��ѧ��ӦΪ��ⱥ��ʳ��ˮ��2NaCl+2H
 O
O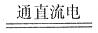 2NaOH+Cl
2NaOH+Cl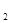
 +H
+H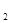
 ��
����5�֣�
��1��ʯ��ʯ��������ԭ��֮һ��������¯�ŷŵķ����������¶Ⱥܸߣ����Һ��д���CO��������¯ú������������������ʯ��ʯ����ʯ�ң�����������������֣���3�֣�
��2��NaCl������������������ռ��ԭ�ϣ�������±�������ɷֵ���ȡ����NaOH��HCl�����Ծ͵�ȡ�ģ���ʱ����ʡȥ�ᴿ���������ᾧ�ȹ��̣�2�֣�
��1��ʯ��ʯ��������ԭ��֮һ��������¯�ŷŵķ����������¶Ⱥܸߣ����Һ��д���CO��������¯ú������������������ʯ��ʯ����ʯ�ң�����������������֣���3�֣�
��2��NaCl������������������ռ��ԭ�ϣ�������±�������ɷֵ���ȡ����NaOH��HCl�����Ծ͵�ȡ�ģ���ʱ����ʡȥ�ᴿ���������ᾧ�ȹ��̣�2�֣�
�����������1������ʯ��ʯ��������ԭ��֮һ��������¯�ŷŵķ����������¶Ⱥܸߣ����Һ��д���CO��������¯ú��������˿�����������ʯ��ʯ��������ʯ�ң�����ʯ��ʯ��ʯ����������������������
��2������NaCl������������������ռ��ԭ�ϣ�������±�������ɷֵ���ȡ����NaOH��HCl�����Ծ͵�ȡ�ģ���ʱ����ʡȥ�ᴿ���������ᾧ�ȹ��̣����Ժ�ˮ���γ����ȼ����������������
��������ֱһ�廯�IJ�ҵģʽ���������ڽ��������ɱ������������̣���߲�Ʒ�����������������ڣ����ֵ����Э���ԡ�ͬʱ�����ô�ֱһ�廯�IJ�ҵģʽ�ɼ��ٶԵ�������Ӧ�̺�ί�������������������������������ͻ�Դ���գ�Ҳ�����ڻ���ϻ��µı�����������������ѧ����ѧϰ��Ȥ������ѧ����ѡ����֪����

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ