��Ŀ����
ij�ᾧˮ����Ļ�ѧʽΪR?nH2O������Է�������ΪM��25��ʱ����a g�þ�������b g H2O��ǡ���γ�V mL������Һ�����б���ʽ��ȷ���ǣ�������
���������ݽᾧˮ�������Է���������������������ʵ���������C=
����������=
��100%��
�ܽ�ȵ���100gˮ���ܽ����ʵ���������=
�����йؼ��㣮
| n |
| V |
| �������� |
| ��Һ���� |
�ܽ�ȵ���100gˮ���ܽ����ʵ���������=
| m |
| V |
����⣺A�����ʵ����ʵ���=
����Һ�����ʵ���Ũ��=
=
mol/L����A����
B�����ʵ�����=a��
=
g�����Ա�����Һ����������=
��100%=
��100%����B��ȷ��
C�����ʵ�����=a��
=
g���ܼ�������=
g+bg���������ܽ��=
=
g����C��ȷ��
D����Һ������=��a+b��g����Һ���ܶ�=
g/mL����D����
��ѡBC��
| a |
| M |
| ||
| V��10-3L |
| 103a |
| MV |
B�����ʵ�����=a��
| M-18n |
| M |
| a(M-18n) |
| M |
| ||
| a+b |
| a(M-18n) |
| M(a+b) |
C�����ʵ�����=a��
| M-18n |
| M |
| a(M-18n) |
| M |
| 18na |
| M |
100��
| ||
|
| 100a(M-18n) |
| 18an+Mb |
D����Һ������=��a+b��g����Һ���ܶ�=
| a+b |
| V |
��ѡBC��
���������⿼�����ʵ��йؼ��㣬��ȷ��ʽ����ʽ�ǽⱾ��ؼ����ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ

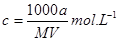
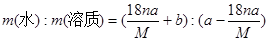
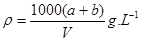
 1000
a(M -18n) mol/L
1000
a(M -18n) mol/L
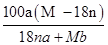 g/100
g H2O
g/100
g H2O g/mL
g/mL