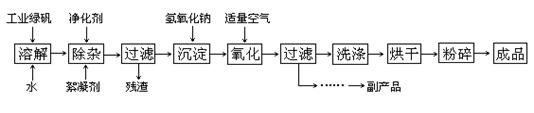
��Ŀ����
(14��) ��ӡ��ʹ�õ�ī����Ҫ�ɷ���Fe3O4����ͼ����������������Fe3O4�Ĺ��գ�

��֪���� ��ҵ�̷���FeSO4�ĺ���Ϊ52.5%�����е����ʲ����뷴Ӧ��
�� Fe(OH)2��2Fe(OH)3��Fe3O4��4H2O
�� 12.16�~1000�~52.5%=6384, 6384��152=42��ش��������⣺
��1�����ij����������� ��
��2��Fe3O4��ϡ���ᷴӦ�����ӷ���ʽ�� �����鷴Ӧ�����Һ�к�Fe3+�ķ��� ��
��3���ڹ��������У�ͨ������������������ʱ�Ļ�ѧ����ʽ�ǣ� ��
��4�������пɻ�õĸ���Ʒ�� ����ȡ�ø���Ʒ�IJ���˳���� ����д��ţ�
a������ b������Ũ�� c����ȴ d���ᾧ e��ϴ��
��5�������������У�����ҵ�̷���Ͷ��������12.16 kg/h��Ϊʹ��Ʒ�ϴ����������������ӦΪ L/h�����跴Ӧ�ڱ�״̬�½��У�������O2ռ20%����
��1��FeO��Fe2O3��Fe3O4 (3��)
��2��3Fe3O4 + 28H����NO3����9Fe3����NO����14H2O (2��) ȡ����Һ�������Թ��У�����������KSCN��Һ������Һ��Ѫ��ɫ����֤����Fe3+ (2��)
��3��4Fe(OH)2��2H2O��O2��4Fe(OH)3 (2��)
��4��Na2SO4��10H2O����â����дNa2SO4Ҳ�ɣ� (1��) bcdae (2��)
��5��784 (2��)
����������1��������Ҫ���ϼ��ǣ�2�ۺͣ�3�ۣ����Գ�����������FeO��Fe2O3��Fe3O4��
��2���������ǿ�����ԣ�Fe3O4�к����������ӣ����Զ��߷���������ԭ��Ӧ��������������NO��ˮ������������һ����KSCN��Һ����������Һ��Ѫ��ɫ��
��3���������������л�ԭ�ԣ����ױ�������������������
��4������ת��ʾ��ͼ��ԭ���غ���жϣ��������������ơ�Ҫʹ��Һ�е���������������Ҫ����Ũ����Ȼ�������ܽ�����¶ȵı仯ͨ����ȴ�ᾧ�����õ������ƾ��壬����ϴ�Ӽ��ɡ�
��5��Ҫʹ��Ʒ�ϴ���������������������Ҫǡ�÷�Ӧ�����������֪��ÿСʱͨ��������������42mol������Fe(OH)2��2Fe(OH)3��Fe3O4��4H2O��֪���������������������������ʵ���֮����1�U2�ģ�������Ҫ����������������28mol�������������� ������ͨ���Ŀ�����7mol��5��22.4L/mol��784L��
������ͨ���Ŀ�����7mol��5��22.4L/mol��784L��

 ������Fe3O4�Ĺ��գ�
������Fe3O4�Ĺ��գ�