��Ŀ����
4�� ̫���ܵ�ذ���ϳ��������⣬����ͭ�������ء����Ȼ�ѧ���ʣ�
̫���ܵ�ذ���ϳ��������⣬����ͭ�������ء����Ȼ�ѧ���ʣ���1����̬��ԭ��M�ܲ�ĵ����Ų�ʽ3s23p2��
��2����һ���������л��軯����й��飮�������ɡ��ṹ����Ӧ���������ƣ������ͨʽΪSinH2n+2�������й��ȡsp3�ӻ���ʽ������ķе�����Է��������Ĺ�ϵ��ͼ��ʾ���������ֱ仯��ԭ���ǹ������Է�������Խ���Ӽ䷶�»���Խǿ��
��3��������ͬΪVIA��Ԫ�أ��������ڵ�Ԫ��������壬��������Ԫ�صĵ�һ������С�����˳��ΪSe��As��Br������Ԫ�ط��ű�ʾ��
��4����̬SeO3���ӵ�VSEPR����Ϊƽ�������Σ���SeO3��Ϊ�ȵ������һ������ΪCO32-��NO3-���ѧʽ����
��5��һ��ͭ��Ͻ�����������������ܶѻ��ṹ���ھ����н�ԭ��λ�ڶ��㣬ͭԭ��λ�����ģ���úϽ��н�ԭ�ӣ�Au������λ��Ϊ1��3�����þ���ľ�������Ϊa pm����úϽ��ܶ�Ϊ$\frac{\frac{197+64��3}{{N}_{A}}}{��a��1{0}^{-10}��{\;}^{3}}$g��cm-3�����г�����ʽ����Ҫ��������������ӵ�������ֵΪNA��
���� ��1������14��Ԫ�أ�����ԭ�Ӻ�������Ų����ɿ���д�������Ų�ʽ��
��2�����ݹ���ķ���ʽ�ó��������ͨʽΪ��SinH2n+2���ֹ������ɡ��ṹ����Ӧ���������ƣ����Թ����й��ȡsp3�ӻ���ʽ������SinH2n+2���Ƿ��Ӿ��壬���Ӿ���ķе�ߵ�ȡ���ڷ��Ӽ��������������Ӽ�����������Է��������Ĵ�С�йأ�
��3�������嶼�ǵ������ڵķǽ���Ԫ�أ�ͬһ����Ԫ���������ҵ�һ�����ܳ�����������ƣ�ע��As�������Ϊ������ṹ��
��4����̬SeO3����������ԭ�ӵļ۲���Ӷ��������жϷ��ӹ��ͣ����ݵȵ�����Ҫ��ԭ��������ͬ���۵�������ͬ��ȷ����
��5�����þ�̯���������ֽ���ԭ�Ӹ���֮�ȣ����ݦ�=$\frac{m}{V}$���㣮
��� �⣺��1������14��Ԫ�أ�����ԭ�Ӻ�������Ų����ɿ���д�������Ų�ʽΪ��1s22s22p63s23p2��M��ĵ����Ų�ʽΪ3s23p2��
�ʴ�Ϊ��3s23p2��
��2�����ݹ���ķ���ʽ�ó��������ͨʽΪ��SinH2n+2���ֹ������ɡ��ṹ����Ӧ���������ƣ����Թ����й��ȡsp3�ӻ���ʽ�����飨SinH2n+2�����Ƿ��Ӿ��壬���Ӿ���ķе�ߵ�ȡ���ڷ��Ӽ��������������Ӽ�����������Է��������Ĵ�С�йأ��������Է�������Խ���Ӽ䷶�»���Խǿ��
�ʴ�Ϊ��SinH2n+2��sp3���������Է�������Խ���Ӽ䷶�»���Խǿ��
��3���顢�����嶼�ǵ������ڵķǽ���Ԫ�أ�ͬһ����Ԫ���������ҵ�һ�����ܳ�����������ƣ�����Asԭ�ӵ�4p�����������ʵ�һ�����ܱ�����Se��As��Br��
�ʴ�Ϊ��Se��As��Br��
��4����̬SeO3����������ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+0}{2}$=3���µ��Ӷԣ����Է��ӹ���Ϊƽ�������Σ��ֵȵ�����Ҫ��ԭ��������ͬ���۵�������ͬ��������SeO3��Ϊ�ȵ������һ������ΪCO32-��NO3-��
�ʴ�Ϊ��ƽ�������Σ�CO32-��NO3-��
��5���ھ����У�Auԭ��λ�ڶ��㣬Cuԭ��λ�����ģ��þ�����Auԭ�Ӹ���=8��$\frac{1}{8}$=1��Cuԭ�Ӹ���=6��$\frac{1}{2}$=3�����ԸúϽ���Auԭ����Cuԭ�Ӹ���֮��=1��3���������V=��a��10-10cm��3��ÿ��������ͭԭ�Ӹ�����3��Auԭ�Ӹ�����1�����=$\frac{\frac{197+64��3}{{N}_{A}}}{��a��1{0}^{-10}��{\;}^{3}}$g•cm-3��
�ʴ�Ϊ��1��3��$\frac{\frac{197+64��3}{{N}_{A}}}{��a��1{0}^{-10}��{\;}^{3}}$g•cm-3��
���� ���⿼�������ʽṹ�����ʣ�Ϊ��Ƶ���㣬���ؿ���ѧ���ķ��������ͼ�����������Ŀ��Ҫ�漰�����Ų�ʽ���縺�ԡ����ӹ��͵��жϡ������ļ���ȣ���Ŀ�Ѷ��еȣ�ע����վ�����йؼ��㷽����

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ������Ϊ�߷��ӻ��������϶࣬�����ж�����-COOH��-NH2 | |
| B�� | ����ʽΪC6H12��̼̼˫�������˵�ϩ������6���칹�� | |
| C�� | CH2=C��C2H5��2��ϵͳ����������Ϊ2-�һ�-1-��ϩ���루CH3��2C=C��CH3��2��Ϊͬϵ�� | |
| D�� | �л���  �ɷ����ӳɡ�ȡ����������������ˮ�ⷴӦ �ɷ����ӳɡ�ȡ����������������ˮ�ⷴӦ |
| A�� | ���ȶ��ԣ�HF��HCl��HBr��HI | B�� | ���ԣ�NaOH��Mg��OH��2��Al��OH��3 | ||
| C�� | ���ԣ�H2SO4��H3PO4��HClO4 | D�� | ԭ�Ӱ뾶��K��Na��Li |
| A�� | �к��������Ƶ�����������=���� | |
| B�� | ���ʵ����ʵ���Ũ�ȣ�������� | |
| C�� | ��ˮϡ��10������Һ��pH��������� | |
| D�� | ˮ�������c��H+����������� |
��NaAlO2��FeCl3���������̷���KNO3��H2S��HCl�൰���ʢ�Na2SO3��CaCl2��
| A�� | �٢ۢݢޢ�� | B�� | �ڢۢܢݢ�� | C�� | �ۢݢߢ� | D�� | �٢ۢݢ� |
 ������˵��������ǣ�������
������˵��������ǣ�������| A�� | M�ķ���ʽΪC7H12 | |
| B�� | N�����е�̼ԭ�Ӳ�������ͬһƽ�� | |
| C�� | M���Է���ȡ����Ӧ�ͼӳɷ�Ӧ | |
| D�� | ��N��һ��̼ԭ����ΪN��ͬϵ���ͬ���칹����22�� |
| A�� | Ba��OH��2•8H2O��NH4Cl��Ӧ | B�� | ������O2��ȼ�շ�Ӧ | ||
| C�� | ����ϡ���� | D�� | ���ȵ�̿��CO2��Ӧ |
| A�� | HF��HCl��HBr��HI | B�� | F2��Cl2��Br2��I2 | ||
| C�� | H2O��H2S��H2Se��H2Te | D�� | CI4��CBr4��CCl4��CF4 |
 ���ң�HOOH��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѣ���ѧ��Baeyer��VilliyerΪ�о�H2O2�Ľṹ����Ʋ����������ʵ�飺a����C2H5OH��ŨH2SO4��Ӧ���ɣ�C2H5��2SO4��ˮ��b�����Ƶõģ�C2H5��2SO4��H2O2��Ӧ��ֻ����A��H2SO4��c�������ɵ�A��H2��Ӧ����֪�÷�Ӧ��H2����ԭ������
���ң�HOOH��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѣ���ѧ��Baeyer��VilliyerΪ�о�H2O2�Ľṹ����Ʋ����������ʵ�飺a����C2H5OH��ŨH2SO4��Ӧ���ɣ�C2H5��2SO4��ˮ��b�����Ƶõģ�C2H5��2SO4��H2O2��Ӧ��ֻ����A��H2SO4��c�������ɵ�A��H2��Ӧ����֪�÷�Ӧ��H2����ԭ������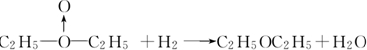 ��
��