��Ŀ����
��15�֣�
��6�֣�����������ɶ�����̼��ˮ�ϳ������ǣ�ͬʱ�ų����������뽫�÷�Ӧ������������Ƴ�һ��ԭ��أ����������������ͽ�̫����ת����˵��ܡ�
��1��д����ԭ��ص��ܷ�Ӧʽ��______________________________________
��2��д���õ�������Խ����зŵ�ĵ缫��Ӧʽ��
������_____________________________________________
������_____________________________________________
��6�֣����˼ά��ѧϰ��ѧ����Ҫ������������Ƿ���ȷ���뾭�ܼ��顣�ڽ������˼ά��ʱ���ܻ�е��ȣ�һ��Ҫע��һЩ���ʵ������ԣ��Է�ֹ��ȳ�����Ľ��ۡ�ƾ���еĻ�ѧ֪ʶ��������Ƚ����ȷ���ǣ��������ţ� �������������д����ȷ�ġ�
������ͬ�����£�Na2CO3�ܽ�ȱ�NaHCO3��
��ȣ�����ͬ�����£�CaCO3�ܽ�ȱ�Ca��HCO3��2��
��ȷ��Ӧ��Ϊ���������ȷ���˴���д����ͬ������ ��
������������Һ��ͨ����CO2��CO2 + ClO- + H2O = HCO3- + HClO
��ȣ������������Һ��ͨ����SO2��SO2 + ClO- +H2O = HSO3- + HClO
��ȷ��Ӧ��Ϊ�� ��
�۸��ݻ��ϼ�Fe3O4�ɱ�ʾΪ��FeO��Fe2O3 ��ȣ�Fe3I8Ҳ�ɱ�ʾΪFeI2��2FeI3
��ȷ��Ӧ��Ϊ�� ��
��CaC2��ˮ�⣺CaC2+2H2O =Ca��OH��2 + C2H2��
��ȣ�Al4C3Ҳ��ˮ�⣺Al4C3 + 12H2O = 4Al��OH��3��+ 3CH4��
��ȷ��Ӧ��Ϊ�� ��
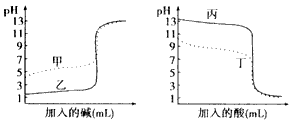
�����֣������£�ijˮ��ҺM�д��ڵ������У�Na+ ��A-��H+��OH-��������ҺM�� pH=3��HA��Һ
��A-��H+��OH-��������ҺM�� pH=3��HA��Һ mL��pH=11��NaOH��Һ
mL��pH=11��NaOH��Һ mL��Ϸ�Ӧ���ã�������˵������ȷ���� ������ĸ����
mL��Ϸ�Ӧ���ã�������˵������ȷ���� ������ĸ����
| A������ҺM�����ԣ�����ҺM��C��H+��+C��OH-��=2��10-7mol��L-1 |
| B����V1=V2������ҺM��pHһ������7 |
| C������ҺM�����ԣ���V1һ������V2 |
| D������ҺM�ʼ��ԣ���V1һ��С��V2 |
��15�֣�
��6�֣�
��1��C6H12O6��6O2 6CO2��6H2O�����֣�
6CO2��6H2O�����֣�
��2��C6H12O6��6H2O��24e��= 6CO2��24H�������֣� 6O2��24e����24H��= 12H2O�����֣�
��6�֣��ܣ�1�֣�
������ͬ�����£�CaCO3�ܽ�ȱ�Ca��HCO3��2С��1�֣�
�� ClO- + SO2 + H2O ==SO42- + Cl- + 2 H+��2�֣���3FeI2��I2��2�֣�
�����֣�AD
����

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
 mL��pH=11��NaOH��Һ
mL��pH=11��NaOH��Һ mL��Ϸ�Ӧ���ã�������˵������ȷ����
������ĸ����
mL��Ϸ�Ӧ���ã�������˵������ȷ����
������ĸ����