��Ŀ����
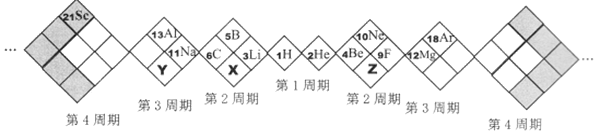
����Ŀ��A��B��C��D��E�Ǻ˵����������������ֶ���������Ԫ�أ�AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ�BԪ�ص�ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ����ࣴ����C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��C��Eͬ���塣
��1��C�����ڱ��е�λ��____________________��
��2����A��B��C����Ԫ���γɵ����ӻ�����Ļ�ѧʽΪ______________________��
��3��Ԫ��C��D��E�γɵ����Ӱ뾶��С��ϵ��___________________�������ӷ��ű�ʾ��.
��4���õ���ʽ��ʾ������D2C���γɹ���____________________________________��
C��D�����γɻ�����D2C2��D2C2���еĻ�ѧ����_____________________________������Ҫ��;��______________________��
��5�����п����м���Ƭ�����ɵ�D��������ٶ�ȫΪD2C2��D2C�Ļ���a�ˣ���VLHCl��Ӧ�����ԣ���HCl��Ũ��ȡֵ��ΧΪ________
���𰸡��ڶ����ڣ�VIA�� NH4NO3 S2-��O2-��Na+ ![]() ���Ӽ����Ǽ��Թ��ۼ� ��������й�������Ư�ȣ����������֣� a/39V��c(HCl)��a/31V
���Ӽ����Ǽ��Թ��ۼ� ��������й�������Ư�ȣ����������֣� a/39V��c(HCl)��a/31V
��������
AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ���AΪHԪ�أ�BԪ�ص�ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3����B������ϼ�Ϊ+5�ۣ�λ�����ڱ��ڢ�A�壬ӦΪNԪ�أ�CԪ��ԭ�ӵ������������ȴ�����4������Cԭ��ֻ����2�����Ӳ㣬����������Ϊ6����CӦΪOԪ�أ�C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C����D�Ļ��ϼ�Ϊ+1�ۣ�ӦΪNaԪ�أ�C��E���壬��EΪSԪ�أ��ݴ˽��
��1��CΪOԪ�أ�ԭ�Ӻ�����2�����Ӳ㣬����������Ϊ6����λ�����ڱ��ڶ����ڣ�VIA�壬
��ˣ�������ȷ���ǣ��ڶ����ڣ�VIA�壻
��2����A��B��C����Ԫ���γɵ����ӻ�����ΪNH4NO3��
��ˣ�������ȷ���ǣ�NH4NO3��
��3��S2-���Ӻ�����3�����Ӳ㣬��O2-��Na+���Ӷ�1�����Ӳ㣬�����Ӱ뾶���O2-��Na+���Ӻ�������Ų���ͬ������2�����Ӳ㣬�˵����Խ�뾶ԽС����뾶O2->Na+�������Ӱ뾶S2-��O2-��Na+��
��ˣ�������ȷ���ǣ�S2-��O2-��Na+��
��4��������D2CΪNa2O��Ϊ���ӻ�����õ���ʽ��ʾ���γɹ���Ϊ![]() ��D2C2ΪNa2O2��Ϊ���ӻ�����,�������Ӽ��ͷǼ��Թ��ۼ���Na2O2����Ҫ��;�к�������й�������Ư�ȣ�
��D2C2ΪNa2O2��Ϊ���ӻ�����,�������Ӽ��ͷǼ��Թ��ۼ���Na2O2����Ҫ��;�к�������й�������Ư�ȣ�
��ˣ�������ȷ���ǣ�![]() �����Ӽ����Ǽ��Թ��ۼ�����������й�������Ư�ȣ�
�����Ӽ����Ǽ��Թ��ۼ�����������й�������Ư�ȣ�
��5����ag����ȫΪNa2O2����VLHCl��Ӧ��������ǡ������NaCl��������Ԫ�غ���Ԫ���غ㣬��HCl��Ũ��Ϊ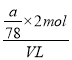 =
=![]() mol/L��ͬ������ag����ȫΪNa2O����VLHCl��Ӧ��������ǡ������NaCl��������Ԫ�غ���Ԫ���غ㣬��HCl��Ũ��Ϊ
mol/L��ͬ������ag����ȫΪNa2O����VLHCl��Ӧ��������ǡ������NaCl��������Ԫ�غ���Ԫ���غ㣬��HCl��Ũ��Ϊ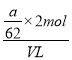 =
=![]() mol/L����HCl��Ũ��ȡֵ��ΧΪa/39V��c(HCl)��a/31V��
mol/L����HCl��Ũ��ȡֵ��ΧΪa/39V��c(HCl)��a/31V��
��ˣ�������ȷ���ǣ�a/39V��c(HCl)��a/31V��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ͼ����ѧ��ѧ�г����ڻ�����ķ�����ᴿװ�ã������װ�ûش����⣺
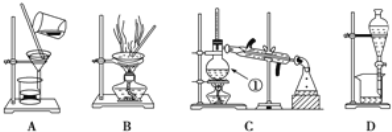
��1��װ��B�б�����������������________��װ��C�Тٵ�������________��
��2��ij�����ƹ����л����������������ʣ������һʵ�鷽�����ȳ�ȥ���ʣ��������������Һ��
ʵ�鷽�����Ƚ�������������ˮ�����Һ��ѡ����ʵ��Լ��Ͳ�����ɱ����и���ʵ�顣
ѡ���Լ� | �� | Na2CO3��Һ | �� |
ʵ����� | �� | �� | ���� |
��������Լ��ٿ�����________���ѧʽ����֤����Һ��SO42-�Ѿ������IJ�����____________������Na2CO3��Һ��Ŀ����_________________________����������Լ�����________���ѧʽ����
��3���������Ǹ�Ч�Ŀ�ű��ҩ��Ϊ��ɫ��״���壬���Ҵ��������п��ܽ⣬��ˮ�м������ܣ��۵�Ϊ156-157�������ȶ��Բ��֪�����ѷе�Ϊ35������ȡ�����ص���Ҫ����Ϊ��
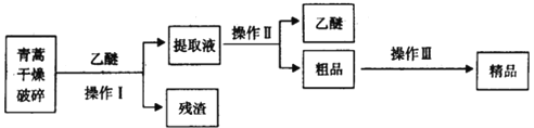
��Ҫ��ʵ����ģ���������գ�����Iѡ���ʵ��װ����____������ţ���������ѡ���ʵ��װ����______������ţ�������������Ҫ�����������________������ĸ����
A.��ˮ�ܽ⣬����Ũ������ȴ�ᾧ������
B.��95%���Ҵ���Ũ�����ᾧ������
C.�������ѽ�����ȡ��Һ