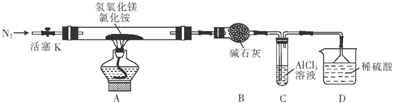
��Ŀ����
14���Ժ���ͭ����Ҫ�ɷ�Ϊͭ������ͭ��Ϊԭ�ϣ���������������ֽ⼼������������ͭ�Ĺ����������£�
��ش��������⣺
��1���ܽ�ʱ����Ũ��һ�������0.25mol•L-1������1000mL�����ᣬ��Ҫ��������Ϊ98%���ܶ�Ϊ1.84g•cm-3Ũ����13.6mL��
��2������ͭ��ͭ�ܽ�ʱ��ֻ���������Σ��÷�Ӧ�����ӷ���ʽΪ4Cu+NO3-+10H+=4Cu2+��+NH4++3H2O��
��3���ܽ�ʱ��Ҫ�����¶���60��70�棬ԭ�����¶ȵ��ܽ��ٶ������¶ȹ�����ηֽ⣮
��4��ˮϴʱ�����ܣ���ܡ����ܡ�����pH=2���������pH=2�����ᣬ������pH=2����������ƷCuCl������Ӧ��
��5����ԭ�����������з�����Ӧ�Ļ�ѧ����ʽΪ��NH4��2SO3+2CuSO4+2NH4Cl+H2O=2��NH4��2SO4+2CuCl��+H2SO4���������Ҫ�ʵ�������ԭ����������Cu2+�Ļ�ԭ���ʣ�ͬʱ���Է�ֹ����Cu+��������������һ�㼴�ɣ���
���� ������������������Ӿ��������ԣ�����������ͭ����Ҫ�ɷ���Cu������CuO����������ͭ�����˺�����Һ�м���������立���������ԭ��Ӧ����CuCl��������Ӧ��2Cu2++SO32-+2Cl-+H2O=2CuCl+SO42-+2H+���õ���CuCl��������ϴ��ˮϴ�������Ҵ�ϴ�ӣ���ɵõ��Ȼ���ͭ��
��1������c=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ������ٸ���ϡ�Ͷ��ɣ�ϡ��ǰ���������ʵ�����ȣ�����������ҪŨ����������
��2������ͭ���������ữ������泥�����������������������Ӿ���ǿ�����ԣ�������Cu����Cu2+����������ӿɱ���ԭ����笠����ӣ�ֻ���������Σ����ݵ��ӵ�ʧ�غ��Լ�ԭ���غ�����ƽ��
��3������������������������Ӿ��������ԣ�������Cu����Cu2+���ܽ��¶�Ӧ������60-70�棬ԭ�����¶ȵ��ܽ��ٶ������¶ȹ�����ηֽ⣻
��4���������ǿ�����ԣ��ܽ�CuCl��������Cu2+��
��5����������ͼ���������ԭ��Ӧ��֪������李��Ȼ�李�����ͭ��ˮ��Ӧ��������李��Ȼ���ͭ������������Ҫ�ʵ��������������Cu2+�Ļ�ԭ���ʣ�ͬʱ���Է�ֹ����Cu+��������������Cu2+��
��� �⣺������������������Ӿ��������ԣ�����������ͭ����Ҫ�ɷ���Cu������CuO����������ͭ�����˺�����Һ�м���������立���������ԭ��Ӧ����CuCl��������Ӧ��2Cu2++SO32-+2Cl-+H2O=2CuCl+SO42-+2H+���õ���CuCl��������ϴ��ˮϴ�������Ҵ�ϴ�ӣ���ɵõ��Ȼ���ͭ��
��1��Ũ��������ʵ���Ũ��c=$\frac{1000�Ѧ�}{M}$=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L������ҪŨ��������ΪV������ϡ�Ͷ��ɣ�ϡ��ǰ���������ʵ�����ȣ���V��18.4mol/L=1000mL��0.25mol•L-1��
��ã�V=13.6mL��
�ʴ�Ϊ��13.6��
��2������ͭ���������ữ������泥�����������������������Ӿ���ǿ�����ԣ�������Cu����Cu2+����������ӿɱ���ԭ����笠����ӣ���Ӧ�����ӷ���ʽΪ��4Cu+NO3-+10H+=4Cu2++NH4++3H2O��
�ʴ�Ϊ��4Cu+NO3-+10H+=4Cu2++NH4++3H2O��
��3��������������������Ӿ��������ԣ�������Cu����Cu2+���ܽ��¶�Ӧ������60-70�棬ԭ�����¶ȵ��ܽ��ٶ������¶ȹ�����ηֽ⣻
�ʴ�Ϊ���¶ȵ��ܽ��ٶ������¶ȹ�����ηֽ⣻
��4���������ǿ�����ԣ��ܽ���ƷCuCl��������Cu2+������ˮϴʱ��������pH=2���������pH=2�����
�ʴ�Ϊ�����ܣ�pH=2����������ƷCuCl������Ӧ��
��5��������ͼ���������ԭ��Ӧ��֪������李��Ȼ�李�����ͭ��ˮ��Ӧ��������李��Ȼ���ͭ�������ѧ����ʽΪ����NH4��2SO3+2CuSO4+2NH4Cl+H2O=2��NH4��2SO4+2CuCl��+H2SO4���������Ҫ�ʵ��������������Cu2+�Ļ�ԭ���ʣ�ͬʱ���Է�ֹ����Cu+������������
�ʴ�Ϊ����NH4��2SO3+2CuSO4+2NH4Cl+H2O=2��NH4��2SO4+2CuCl��+H2SO4�����Cu2+�Ļ�ԭ���ʣ�ͬʱ���Է�ֹ����Cu+������������
���� ������Ҫ����Թ�ҵ���̵ķ����������Ʊ�ԭ���ķ����жϣ������ѶȽϴ�ע��������ԭ��Ӧԭ�������ã�

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�| A�� | ���� | B�� | ����������Һ | C�� | ��ˮ | D�� | ˮ |
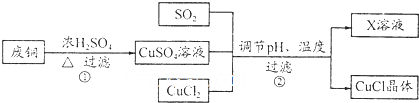
��l��д�����̢ٵ���Ҫ��ѧ����ʽCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��2�������±�������CuCl����ʱ�����pHΪ2����pH�ϴ�ʱ��CuCI������ʽϵͣ���ԭ����pH����Cu2+ˮ����ʧ���ӣ�
| pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| CuCl���ʣ�%�� | 70 | 90 | 82 | 78 | 75 | 72 | 70 |
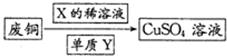
��4��Ϊ�˸���������ɫ��ѧ˼�룬����������·���������һ���ɽ�����Xϡ��Һ���ڷ�ͭ�Ĵ�������ͼ��ʾ������Y����ΪO2���ѧʽ����
������������Cu��ŨH2SO4��Ӧ����SO2��CuSO4���ڢ���CuCl���Ʊ��������ϲ���Ҫ������Ҫ������Ҫ�������ⲹ��SO2��ԭ���Ƿ�Ӧ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O�����ɵ�CuSO4��SO2�����ʵ���֮��Ϊ1��1������Ӧ��CuSO4+CuCl2+SO2+2H2O�T2Cucl��+2H2SO4��Ҫ��CuSO4��SO2�����ʵ���֮��Ҳǡ��Ϊ1��1�����������ϲ���Ҫ����SO2����ϻ�ѧ����ʽ�ش𣩣�
| ҩƷ���� | �۵�/�� | �е�/�� | �ܶ�g/cm3 | �ܽ��� |
| ������ ��CH3CH2CH2OH�� | -89.5 | 117.7 | 0.8098 | ����ˮ������Ũ���� |
| l-�嶡�� ��CH3CH2CH2CH2Br�� | -112.4 | 101.6 | 1.2760 | ������ˮ��Ũ���� |
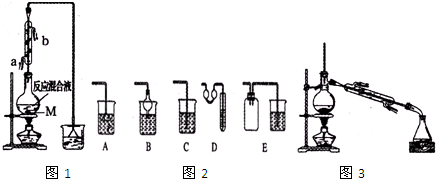
��1����Ӧװ���л���Ҫ���˹���M������M��Ŀ���Ƿ�ֹҺ�屩�У������������Ϊ1��1���������õIJ�������Ϊbcd��a����ƽ��b����Ͳ�� C����������d���ձ�
��2�������ܵĽ�ˮ���ǣ��ˣ�����ѡ���ԭ�����ܸ����������
��3������ͼ2װ���У��ܴ���ͼ1�������ռ�װ�����B��D��E��
��4������Ũ�������ʵ�飬�л����л�����ػ�ɫ����ȥ�������ʵ���ȷ������d
a��������������b������������Һϴ��
c�����ķջ�̼��ȡ d��������������Һϴ��
��5���Ʊ���Ʒ�����õ��Ĵ�1һ�Ķ���������Ũ���ᡢˮ��10д̼���ơ�ˮϴ�Ӻ������ˮץ���ƽ��и��Ȼ���ٽ�1-�Ķ��鰴ͼ3װ������
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��101.6�����ң�����1һ�Ķ��龫Ʒ�ʹ�Ʒ��һ�ַ����Dz��۷е㣮
��ʵ���Ƶõ�1һ�Ķ��������Ϊ10.895g������������ת����Ϊ72.6%��������3λС����
| A�� | �������е����Ų�ʽ��ԭ���У���1s22s22p63s23p2����1s22s22p3����1s22s22p2����1s22s22p63s23p1��ԭ�Ӱ뾶�ܣ��٣��ۣ��� | |
| B�� | �������м۵����Ų�ʽ��ԭ���У���1s22s22p63s2����1s22s22p63s23p1����1s22s2 2p3����1s22s22p4����һ�����ܣ��ۣ��ܣ��٣��� | |
| C�� | ��Na��K��Rb����N��P��As����O��S��Se ����Na��P��Cl��Ԫ�صĵ縺����ԭ������������������Ǣ� | |
| D�� | ijԪ����̬��̬ԭ�ӵ������ܣ�kJ•mol-1���ֱ�Ϊ738��1451��7733��10540��13630��17 995��21703��������������Ӧʱ�������ɵ���������X3+ |
| A�� | ��������Լ����Ǽ��Լ� | B�� | �������������Լ� | ||
| C�� | �����������Ǽ��Լ� | D�� | ������Ǽ��Լ������Լ� |
| A�� | 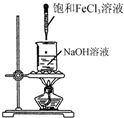 �Ʊ������������� | B�� | 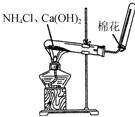 ��ȡNH3 | ||
| C�� | 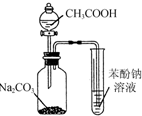 ֤��H2CO3����ǿ�ڱ��� | D�� | 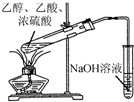 �Ʊ��������� |
| A�� | K+��Cl-��CO32-��Na+ | B�� | H+��Ba2+��Fe3+��S2- | ||
| C�� | NH4+��SO42-��K+��OH- | D�� | H+��NO3-��Na+��SiO32- |