��Ŀ����
���ᾧ�壨2C2O4��2H2O�������ֽ⣺�ٽ��ֽ������ͨ�����������ձ������ֲ���ˮ�飩����Ȼ�����ɵ�����ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǡ��ס���ͬѧ��Ϊ��
|
�ң�H2C2O4��2H2O
| A������֤ʣ�������Ƿ��ȼ��ȼ��ʱ�������ɫ |
| B����ʣ�����廹ԭ�ȵ�CuO��ĩ���۲������ɫ�ı仯 |
| C����ʣ������ͨ����ˮ�У�����ˮ�Ƿ���ɫ |
| D����ʣ����������ȼ�գ�������ˮ����ͭ����ȼ�ղ��� |
D
����

��ϰ��ϵ�д�
 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
�����Ŀ
|
|
�ң�H2C2O4��2H2O CO��+CO2��+3H2OΪ�жϼס��Һ�����ȷ���ڢ�֮���貹����ʵ��Ϊ �� ��
A������֤ʣ�������Ƿ��ȼ��ȼ��ʱ�������ɫ
B����ʣ�����廹ԭ�ȵ�CuO��ĩ���۲������ɫ�ı仯
C����ʣ������ͨ����ˮ�У�����ˮ�Ƿ���ɫ
D����ʣ����������ȼ�գ�������ˮ����ͭ����ȼ�ղ���
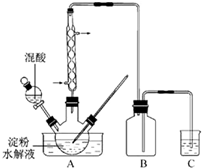 ��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ��
��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ��