��Ŀ����
(10��)��1��AgNO3��ˮ��Һ�� ����ᡱ�����С���������ԣ�ԭ���ǣ������ӷ���ʽ��ʾ���� ������������������ ��
��2���Ȼ���ˮ��Һ�� �� ������ᡱ�����С���������ԣ�ԭ���ǣ������ӷ���ʽ��
ʾ���� ;
��AlCl3��Һ���ɣ����գ����õ�����Ҫ��������� ��
(3) ��֪MgCl2��6H2O�����ڿ����м���ʱ���ͷŲ��ֽᾧˮ��ͬʱ����Mg(OH)Cl����ʽ�Ȼ�þ��������MgO�������ǹ���MgCl2��6H2O���ۺ�Ӧ�ã�
��ش��������⣺
������ͼ��������Ӧ�������_______________�����м��ȡ�
��Mg(OH)2������������ܽ�ƽ�⣺Mg(OH)2(��) ![]() Mg2����2OH��������ϵ�м��루��������ֲ�ͬ�������ʣ�_____________________________________________________ ������Mg(OH)2�ܽ⡣
Mg2����2OH��������ϵ�м��루��������ֲ�ͬ�������ʣ�_____________________________________________________ ������Mg(OH)2�ܽ⡣
����֪AnBm�����ӻ���c (Am��) n ��c (Bn��) m��ʽ��c (Am��) n��c (Bn��) m��ʾ���ӵ����ʵ���Ũ�ȣ�������ʱ�����Mg(OH)2������Һ��pHֵΪ11�������ӻ�Ϊ___________________��
��1���ᣬ������Ag�� �� H2O ![]() AgOH�� H��
AgOH�� H��
��2���ᣬ������Al3����3H2O ![]() Al(OH)3��3H���� ������Al2O3 ��
Al(OH)3��3H���� ������Al2O3 ��
(3) ��HCl���� ���ᡢˮ���������塢����NH4Cl�ȣ���ѡ���֣� ��ÿ��1�֣�
��5��10-10��2�֣�
����:��1��AgNO3��ǿ�������Σ�ˮ�������ԡ�
��2���Ȼ���Ҳ��ǿ�������Σ�ˮ�������ԡ�������ˮ�����������������Ȼ��⣬ˮ�����ȣ����ȴٽ�ˮ�⡣ͬʱ�Ȼ���Ļӷ�����һ���ٽ�ˮ�⣬�������յõ������������������յõ���������
��3���Ȼ�þˮ�������ԣ�����Ҫ�õ��Ȼ�þ���壬�ڼ���ʱ��Ҫ��ֹˮ�⣬���������������м��ȼ��ɡ�����������þ���ܽ�ƽ���֪��Ҫ�ٽ��ܽ⣬�ͱ���ʹƽ��������Ӧ�����ƶ������Կ���ͨ������OH����þ������ʵ�֡��������ӻ������ĸ����֪�����ӻ������ǵ���������ӵ�Ũ�ȵ���֮����Mg(OH)2������Һ��pHֵΪ11����OH����Ũ��Ϊ10��3mol/L����þ����Ũ��Ϊ5��10��4mol/L������������þ�����ӻ�����Ϊ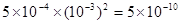 ��
��

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� Ag++2CN - == [Ag(CN)2] -
Ag++2CN - == [Ag(CN)2] -