��Ŀ����
(9��)ij�ǽ�������A������ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺
��1����A������Ϊ����ɫ���壬B���д̼�����ζ����ɫ���塣
��A��D�Ļ�ѧʽ�ֱ�Ϊ��A D
�ڹ�ҵ�����д����ŷŵ�B���屻��ˮ���պ��γ� ����Ⱦ������
��д��B��C��Ӧ�Ļ�ѧ����ʽ��
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��A�ĵ���ʽΪ ��C�Ļ�ѧʽΪ
��д��C��D��Ӧ�Ļ�ѧ����ʽ��
�� A S D H2SO4 �� ���꣨����ʽÿ��2�֣�����ÿ��1�ֹ�9�֣�
��2SO2+O2 2SO3
2SO3
��2���� 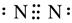 NO2 ��3NO2+H2O=2HNO3+NO
NO2 ��3NO2+H2O=2HNO3+NO
����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


