��Ŀ����
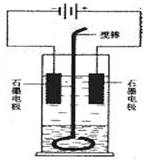
(8��)��ͼ�е缫a��bΪFe�����AgƬ���缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�����Ϲ��ռ���336ml(��״��)���塣�ش�
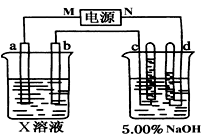
(1)ֱ����Դ�У�MΪ_________________����
(2)����Fe����϶���һ����Ag��
��Fe���ӦΪ_________����(��a��b)
��Fe�������_________g��
(3)X��ҺΪ____________����Ũ��____________��(�������С�����䡱)
(4)��NaOH��Һ������������5.00%��Ϊ5.02%����ʽ����ԭNaOH��Һ��������

(1)ֱ����Դ�У�MΪ_________________����
(2)����Fe����϶���һ����Ag��
��Fe���ӦΪ_________����(��a��b)
��Fe�������_________g��
(3)X��ҺΪ____________����Ũ��____________��(�������С�����䡱)
(4)��NaOH��Һ������������5.00%��Ϊ5.02%����ʽ����ԭNaOH��Һ��������
��1���� ��2��b 2.16g AgNO3 (aq) ���� (4)45.18��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ
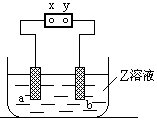
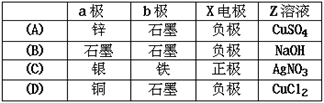

 ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���
ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ��� Cu2(OH)2CO3
Cu2(OH)2CO3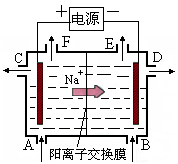
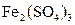 ��
�� �Ļ��Һ������֪
�Ļ��Һ������֪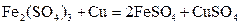 ������˵����ȷ����
������˵����ȷ����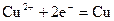 ������
������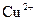 ����ʱ
����ʱ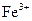 ���ŵ�
���ŵ�
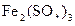 ��
�� ��������
��������