��Ŀ����
���ס�������������ͬ�������壬�ڿ�����ȼ�յõ����������������ʱ����P4O6����������ʱ����P4O10��
��1����֪298 Kʱ���ס�������ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�ΪP4��s�����ף���5O2��g��=P4O10��s����H1����2 983.2 kJ��mol��1��P��s�����ף��� O2��g��=
O2��g��=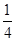 P4O10��s����H2����738.5 kJ��mol��1������¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ________________��
P4O10��s����H2����738.5 kJ��mol��1������¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ________________��
��2����֪298 Kʱ���ײ���ȫȼ�յ��Ȼ�ѧ����ʽΪP4��s�����ף���3O2��g��=P4O6��s����H����1 638 kJ��mol��1����ij�ܱ������м���62 g����50.4 L��������״��������������ʹ֮ǡ����ȫ��Ӧ�������õ���P4O10��P4O6�����ʵ���֮��Ϊ________����Ӧ�����зų�������Ϊ________��
��3����֪����PCl3�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ��mol��1����P��P 198��Cl��Cl 243��P��Cl 331��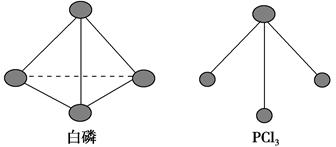
��ӦP4��s�����ף���6Cl2��g��=4PCl3��s���ķ�Ӧ�Ȧ�H��________��
��1��P4��s�����ף�=4P��s�����ף���H����29.2 kJ��mol��1
��2��3��1��1 323.45 kJ
��3����1 326 kJ��mol��1
����

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д������������������йط�Ӧ���ʱ䣺
��1����֪��Ti(s)��2Cl2(g)=TiCl4(l)��H����804��2 kJ��mol��1
2Na(s)��Cl2(g)=2NaCl(s)����H����882��0 kJ��mol��1
Na(s)=Na(l)����H����2��6 kJ��mol��1
��ӦTiCl4(l)��4Na(l)=Ti(s)��4NaCl(s)�Ħ�H��________ kJ��mol��1��
��2����֪���з�Ӧ��ֵ��
| ��Ӧ��� | ��ѧ��Ӧ | ��Ӧ�� |
| �� | Fe2O3(s)��3CO(g)= 2Fe(s)��3CO2(g) | ��H1����26��7 kJ��mol��1 |
| �� | 3Fe2O3(s)��CO(g)=2Fe3O4(s)��CO2(g) | ��H2����50��8 kJ��mol�� |
| �� | Fe3O4(s)��CO(g)=3FeO(s)��CO2(g) | ��H3����36��5 kJ��mol��1 |
| �� | FeO(s)��CO(g)=Fe(s)��CO2(g) | ��H4 |
��Ӧ�ܵĦ�H4��____________ kJ��mol��1��
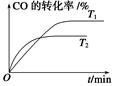
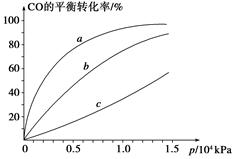

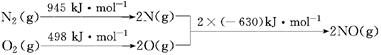
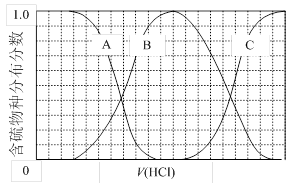
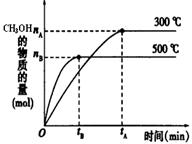
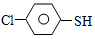 �����ȩ���Ȼ��������ʵ���֮��1:1:1��Ӧ���ɻ��һ��ɱ����м���X��H2O��
�����ȩ���Ȼ��������ʵ���֮��1:1:1��Ӧ���ɻ��һ��ɱ����м���X��H2O��
 CH3OH��g���ﵽ��ѧƽ��״̬��
CH3OH��g���ﵽ��ѧƽ��״̬��