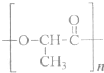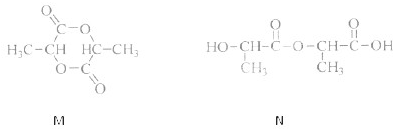��Ŀ����
��������ɸ�������ɺ����ʽ��з��ࣺ
��1��������ʾ�����ʷ����������______��
��2����Na��K��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��Тڢۢ��森��������дһ����ѧʽ���ɣ�
��3������10�����ʣ���ˮ �ڿ��� ��ͭ˿ �ܶ�����̼ ������ ����������
������ ��Fe��OH��3���� ��̼�����[Ca��HCO3��2]��NH3
���ڵ���ʵ���______�� ���ڷǵ���ʵ���______������ţ�
��4����Ҫ��д�����з�Ӧ�����ӷ���ʽ��
��п��ϡ���ᷴӦ______
������������Һ��ϡ���ᷴӦ______
��MgO�μ�ϡ����______��

��1��������ʾ�����ʷ����������______��
��2����Na��K��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��Тڢۢ��森��������дһ����ѧʽ���ɣ�
| ������� | �� | �� | �� | ������ | �⻯�� |
| ��ѧʽ | ��HCl ��______ |
��______ ��Ba��OH��2 |
��Na2CO3 ��______ |
��CO2 ��Na2O |
��NH3 ��H2O |
������ ��Fe��OH��3���� ��̼�����[Ca��HCO3��2]��NH3
���ڵ���ʵ���______�� ���ڷǵ���ʵ���______������ţ�
��4����Ҫ��д�����з�Ӧ�����ӷ���ʽ��
��п��ϡ���ᷴӦ______
������������Һ��ϡ���ᷴӦ______
��MgO�μ�ϡ����______��
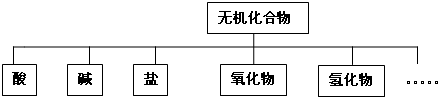
��1����״���෨��һ�ֺ�����ķ��෨�����ղ�Σ�һ��һ�����֣�����һ�ô�������Ҷ��֦���ˡ�����ͼʾ����������״ͼ���ʴ�Ϊ����״���෨��
��2����Na��K��H��O��S��N������Ԫ�����������ֻ�����Ԫ�ؿ�������У�H2SO4��HNO3�����У�NaOH��KOH��NH3?H2O�����У�NaNO3��KNO3��Na2SO4��K2SO4��NH4NO3�ȣ�
�ʴ�Ϊ����H2SO4��HNO3 ��NaOH��KOH����NaNO3��KNO3��K2SO4��Na2SO4��
��3�����ڵ���ʵ��Ǣ١��ݡ��ޡ�� ���ڷǵ���ʵ��Ǣܡ��⣻�ʴ�Ϊ���١��ݡ��ޡ���ܡ��⣻
��4����п��ϡ���ᷴӦ�����ӷ���ʽ��Zn+2H+�TZn2++H2����
������������Һ��ϡ���ᷴӦ�����ӷ���ʽ��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
��MgO�μ�ϡ��������ӷ���ʽ��MgO+2H+�TMg2++H2O��
�ʴ�Ϊ����Zn+2H+�TZn2++H2����Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��MgO+2H+�TMg2++H2O��
��2����Na��K��H��O��S��N������Ԫ�����������ֻ�����Ԫ�ؿ�������У�H2SO4��HNO3�����У�NaOH��KOH��NH3?H2O�����У�NaNO3��KNO3��Na2SO4��K2SO4��NH4NO3�ȣ�
�ʴ�Ϊ����H2SO4��HNO3 ��NaOH��KOH����NaNO3��KNO3��K2SO4��Na2SO4��
��3�����ڵ���ʵ��Ǣ١��ݡ��ޡ�� ���ڷǵ���ʵ��Ǣܡ��⣻�ʴ�Ϊ���١��ݡ��ޡ���ܡ��⣻
��4����п��ϡ���ᷴӦ�����ӷ���ʽ��Zn+2H+�TZn2++H2����
������������Һ��ϡ���ᷴӦ�����ӷ���ʽ��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��
��MgO�μ�ϡ��������ӷ���ʽ��MgO+2H+�TMg2++H2O��
�ʴ�Ϊ����Zn+2H+�TZn2++H2����Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O��MgO+2H+�TMg2++H2O��

��ϰ��ϵ�д�
�����Ŀ