��Ŀ����
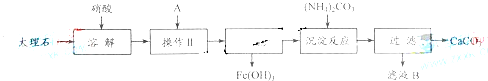
ʵ�����ô���ʯ��ԭ����ȡ��ȫ����ɱ�����������ơ�����ʯ����Ҫ���������������������ᴿ����ʯ��ʵ�鲽�裺
![]()

![]() ��1���ܽ����ʯʱ�����������ͬ�����ԭ����_______________________________��
��1���ܽ����ʯʱ�����������ͬ�����ԭ����_______________________________��
![]() ��2���������Ŀ����______________����ҺA�������ǹ��ۻ������___________��
��2���������Ŀ����______________����ҺA�������ǹ��ۻ������___________��
![]() ��3��д��������Һ���Ƿ������ӷ���ʽ��_____________________________________
��3��д��������Һ���Ƿ������ӷ���ʽ��_____________________________________
![]() ______________________________________________________________________��
______________________________________________________________________��
![]() ��4��д������̼�����������Ӧ�����ӷ���ʽ��________________________________
��4��д������̼�����������Ӧ�����ӷ���ʽ��________________________________
![]() д����ҺB��һ����;��_________________________
д����ҺB��һ����;��_________________________
![]() ��5��
��5��![]() ��һ�㺬
��һ�㺬![]() ����������ʾ���
����������ʾ���![]() ����������ʵ����ơ�
����������ʵ����ơ�
![]() �Լ����������Ʊ���Һ���������Һ����̪ ������������ƽ����ƿ���ζ���
�Լ����������Ʊ���Һ���������Һ����̪ ������������ƽ����ƿ���ζ���
![]() ʵ�鲽�裺
ʵ�鲽�裺
![]() ��_______���ڼ���_________________���ۼ����̪�����������Ʊ���Һ�ζ���
��_______���ڼ���_________________���ۼ����̪�����������Ʊ���Һ�ζ���
![]() ��6���������ϵζ�ʱ���ñ���Һ20.00mLǡ����ȫ��Ӧ��ʵ�ʲ����й�����Σ�1mL��ҺΪ25�Σ�����������Ϊ______________
��6���������ϵζ�ʱ���ñ���Һ20.00mLǡ����ȫ��Ӧ��ʵ�ʲ����й�����Σ�1mL��ҺΪ25�Σ�����������Ϊ______________
![]()
![]()
�𰸣�
![]() ��1�����������ˮ
��1�����������ˮ
![]() ��2����ȥ��Һ��
��2����ȥ��Һ��![]() ��ˮ
��ˮ
![]() ��3��ȡ������Һ�������м������軯����Һ����Һ�����ɫ��˵����Һ�в���
��3��ȡ������Һ�������м������軯����Һ����Һ�����ɫ��˵����Һ�в���![]() ��
��![]() ��ȡ������Һ�������м������軯����Һ����Һ���ɫ��˵����Һ�к�
��ȡ������Һ�������м������軯����Һ����Һ���ɫ��˵����Һ�к�![]()
![]() ��4��
��4��![]() �����ʣ����������֣�
�����ʣ����������֣�
![]() ��5������ �����ı�����
��5������ �����ı�����
![]() ��6��0.1%
��6��0.1%
����������2������ʯ�������������Ca(NO3)2��Fe(NO3)3����ͨ������Һ�м�CaCO3��ˮ�ķ�������pH��ʹFe3����Fe(OH)3����ʽ��ȥ[Fe(OH)3�������Ի����¾��ܳ���]������ΪҪ����ҺA�������ǹ��ۻ��������AӦΪ��ˮ��(4)��ҺB��������NH4NO3�������������ʡ�
��6����ε����Ϊ1/50mL����������Ϊ ��100%=0.1%
��100%=0.1%
![]()
![]()