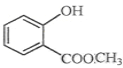
��Ŀ����
����Ŀ��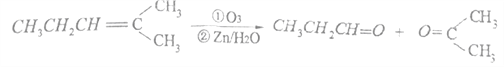
������Ӧ�������ƶ�ϩ���Ľṹ��ij��ϩ��A���Է�������ͼʾ��ת�����ش���������
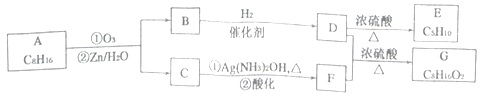
��1��B�ķ���ʽΪ_____________��D�к��й����ŵ�����________________��
��2��C��F�в������Ļ�ѧ��Ӧ����ʽ______________���÷�Ӧ�ķ�Ӧ������___________��
��3��B��������Ӧ��D��Ũ��������¼��ȿɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E(�����������칹)����A�Ľṹ��ʽΪ________________��
��4��G��ͬ���칹���У���������Ҫ��Ľṹ��__________��(�����������칹)��
������������������������ˮ���ܵõ���Է�������Ϊ74���л���
��5����֪��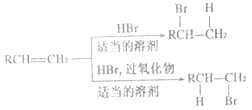 ����д����C�Ʊ�2�������ĺϳ�·��_________________��
����д����C�Ʊ�2�������ĺϳ�·��_________________��
���𰸡�C5H10O �ǻ� CH3CH2CHO + 2Ag(NH3)2OH![]() CH3CH2COONH4+3NH3+2Ag��+H2O ������Ӧ
CH3CH2COONH4+3NH3+2Ag��+H2O ������Ӧ ![]() 16
16 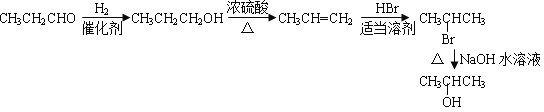
��������
�����������ͼ����ϩ��A������ΪB��C��C����3��̼ԭ�ӣ�C�ܱ�������Һ����������C�DZ�ȩ�� F�DZ��B����5��̼ԭ�ӣ�B��������Ӧ��B��ͪ��B�����������ӳɷ�Ӧ����D��D�Ǵ���D��Ũ��������¼��ȷ�����ȥ��Ӧ���õ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ�����ʣ�E��CH3CH=CHCH2CH3��D��![]() ��B��
��B��![]() ����A��
����A��![]() ��G��
��G��![]() ��
��
�������������Ϸ�������1��B��![]() ��B�ķ���ʽΪC5H10O��D��
��B�ķ���ʽΪC5H10O��D��![]() �����й����ŵ������ǻ���
�����й����ŵ������ǻ���
��2����ȩ��������Һ�����ķ�Ӧ����ʽ��CH3CH2CHO + 2Ag(NH3)2OH![]() CH3CH2COONH4+3NH3+2Ag��+H2O���÷�Ӧ�ķ�Ӧ������������Ӧ��
CH3CH2COONH4+3NH3+2Ag��+H2O���÷�Ӧ�ķ�Ӧ������������Ӧ��
��3��A�Ľṹ��ʽΪ![]() ��
��
��4��G��![]() �����������ຬ��������������������ˮ���ܵõ���Է�������Ϊ74���л�����л�����
�����������ຬ��������������������ˮ���ܵõ���Է�������Ϊ74���л�����л�����![]() ��
��![]() ������ܵĽṹ��ʽ��
������ܵĽṹ��ʽ��![]() ��
��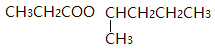 ��
��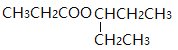 ��
�� ��
�� ��
��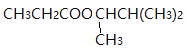 ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ����16�֣�
����16�֣�
��5����ȩ�����������ӳɷ�Ӧ����1-������1-������Ũ���������·�����ȥ��Ӧ���ɱ�ϩ����ϩ���廯�����ʵ����ܼ��з�Ӧ����2-����飬2-���������������ˮ��Һ��ˮ������2���������ɱ�ȩ�Ʊ�2�������ĺϳ�·����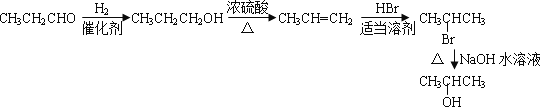 ��
��

 ��У����ϵ�д�
��У����ϵ�д�