��Ŀ����
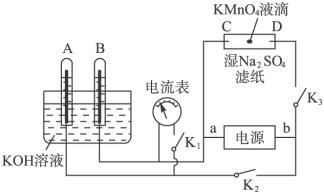
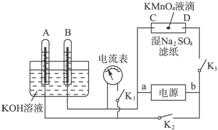
��1������������ɴ�ʹ�õ��Ǽ�������ȼ�ϵ�أ������������������������������������������NaOH��Һ����д���÷�Ӧ�����ĵ缫��Ӧʽ��
������______��������______���ܷ�Ӧ��______��
��2����ͼΪֱ����Դ���ϡNa2SO4ˮ��Һ��װ�ã�ͨ�����ʯī�缫a��b�����ֱ�μ�һ��ʯ����Һ��
��a�缫�ϵĵ缫��ӦʽΪ______��
����b�������۲쵽��������______��
�۵��һ��ʱ���������______����ʹ�������Һ�ָ���ԭ����Ũ�ȣ�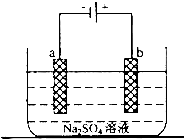
������______��������______���ܷ�Ӧ��______��
��2����ͼΪֱ����Դ���ϡNa2SO4ˮ��Һ��װ�ã�ͨ�����ʯī�缫a��b�����ֱ�μ�һ��ʯ����Һ��
��a�缫�ϵĵ缫��ӦʽΪ______��
����b�������۲쵽��������______��
�۵��һ��ʱ���������______����ʹ�������Һ�ָ���ԭ����Ũ�ȣ�

��1������������ɴ�ʹ�õ��Ǽ�������ȼ�ϵ�أ������������������������������������������NaOH��Һ�������缫���������õ����ӷ�����ԭ��Ӧ�缫��ӦΪ��O2+2H2O+4e-=4OH-������������ʧ���ӷ���������Ӧ���缫��ӦΪ��2H2+4OH--4e-=4H2O����ص��ܷ�ӦΪ��2H2+O2=2H2O��
�ʴ�Ϊ��O2+2H2O+4e-=4OH-��2H2+4OH--4e-=4H2O��2H2+O2=2H2O��
��2����ͼΪֱ����Դ���ϡNa2SO4ˮ��Һ��װ�ã�ͨ�����ʯī�缫a��b�����ֱ�μ�һ��ʯ����Һ�����ݵ��صĹ���ԭ���ж�aΪ��������Һ�е������ӵõ����ӷ�����ԭ��Ӧ���缫��ӦΪ��2H++2e-=H2����bΪ������Һ�е�����������ʧ���ӷ���������Ӧ��4OH--4e-=2H2O+O2�������������Ӽ��٣������������ٽ�ˮ�ĵ��룬��Һ��������Ũ����������ʯ����Һ���ɫ���������ǵ缫��ˮ���ָ�ԭ��ҺŨ����Ҫ����ˮ��
�ʴ�Ϊ����2H++2e-=H2���� ��������ð������Һ�Ժ�ɫ�� ��H2O��
�ʴ�Ϊ��O2+2H2O+4e-=4OH-��2H2+4OH--4e-=4H2O��2H2+O2=2H2O��
��2����ͼΪֱ����Դ���ϡNa2SO4ˮ��Һ��װ�ã�ͨ�����ʯī�缫a��b�����ֱ�μ�һ��ʯ����Һ�����ݵ��صĹ���ԭ���ж�aΪ��������Һ�е������ӵõ����ӷ�����ԭ��Ӧ���缫��ӦΪ��2H++2e-=H2����bΪ������Һ�е�����������ʧ���ӷ���������Ӧ��4OH--4e-=2H2O+O2�������������Ӽ��٣������������ٽ�ˮ�ĵ��룬��Һ��������Ũ����������ʯ����Һ���ɫ���������ǵ缫��ˮ���ָ�ԭ��ҺŨ����Ҫ����ˮ��
�ʴ�Ϊ����2H++2e-=H2���� ��������ð������Һ�Ժ�ɫ�� ��H2O��

��ϰ��ϵ�д�
 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ
 ��1������������ɴ�ʹ�õ��Ǽ�������ȼ�ϵ�أ������������������������������������������NaOH��Һ����д���÷�Ӧ�����ĵ缫��Ӧʽ��
��1������������ɴ�ʹ�õ��Ǽ�������ȼ�ϵ�أ������������������������������������������NaOH��Һ����д���÷�Ӧ�����ĵ缫��Ӧʽ��