��Ŀ����
��֪A��B��C��Ϊ�������ʣ�����A�ǽ�����B��C�Ƿǽ�������һ���������ת����ϵ����ͼ��ʾ����Ӧ�����Ͳ��ֲ�����ʡ�ԣ���

��ش��������⣺
��1���������£�B��C��Ϊ���壬DΪ��ɫ���Թ��壬��C�Ļ�ѧʽΪ �� A��E��Ӧ����D�Ļ�ѧ����ʽΪ ��
��2���������£�BΪ���壬CΪ��ɫ�����ĩ�������A��ԭ�ӽṹʾ��ͼΪ �� A��E��һ�������·�Ӧ����D�Ļ�ѧ����ʽΪ ��

��ش��������⣺
��1���������£�B��C��Ϊ���壬DΪ��ɫ���Թ��壬��C�Ļ�ѧʽΪ �� A��E��Ӧ����D�Ļ�ѧ����ʽΪ ��
��2���������£�BΪ���壬CΪ��ɫ�����ĩ�������A��ԭ�ӽṹʾ��ͼΪ �� A��E��һ�������·�Ӧ����D�Ļ�ѧ����ʽΪ ��
��1��H2 ��1�֣�
3Fe + 4H2O��g�� Fe3O4 + 4H2 ��2�֣�
Fe3O4 + 4H2 ��2�֣�
��2��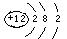 ��1�֣� 2Mg + CO2
��1�֣� 2Mg + CO2 2MgO + C ��2�֣�
2MgO + C ��2�֣�
3Fe + 4H2O��g��
 Fe3O4 + 4H2 ��2�֣�
Fe3O4 + 4H2 ��2�֣���2��
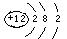 ��1�֣� 2Mg + CO2
��1�֣� 2Mg + CO2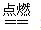 2MgO + C ��2�֣�
2MgO + C ��2�֣���

��ϰ��ϵ�д�
�����Ŀ
 �ų�������һ���Է��Ļ�ѧ�仯���̣����߶���Σ���ܴ�
�ų�������һ���Է��Ļ�ѧ�仯���̣����߶���Σ���ܴ� U��
U�� U����������ͬ��������ͬ��ͬ�ֺ���
U����������ͬ��������ͬ��ͬ�ֺ��� ��D��F��N��OΪ��ɫ���壬E���³�ѹ��Ϊ��ɫ��ζ��Һ�壬N��H��LΪ���г����ĵ��ʣ�IΪ��������ǿ�ᣬM����ɫ��ӦΪ��ɫ����Ӧ�ٳ���������F�ļ��顣
��D��F��N��OΪ��ɫ���壬E���³�ѹ��Ϊ��ɫ��ζ��Һ�壬N��H��LΪ���г����ĵ��ʣ�IΪ��������ǿ�ᣬM����ɫ��ӦΪ��ɫ����Ӧ�ٳ���������F�ļ��顣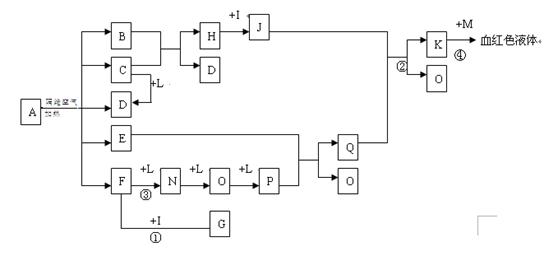
 ______��
______��
 _______��
_______��