��Ŀ����
���ںϳɰ���ӦN2(g)��3H2(g) 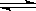 2NH3(g)��H=-92 kJ/mol������˵����ȷ���ǣ� ��
2NH3(g)��H=-92 kJ/mol������˵����ȷ���ǣ� ��
A������ʼ����2molN2��6molH2������Ӧ����ƽ��ʱN2��H2��ת������ͬ
B���÷�Ӧ���ø������������������NH3�IJ��ʣ��Ӷ��������Ч��
C. ����ʼ����2molN2��6molH2������Ӧ��ƽ��ʱ������46kJ����N2��ƽ��ת����Ϊ50��
D����ƽ��ʱ���������������䣬ѹ�������������ƽ�������ƶ���N2��Ũ�ȼ�С��NH3��Ũ������
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


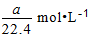 B.
B.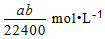
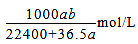 D.
D.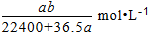
 2NO2(g)������ȴ�����¶ȵ����߶���С��ij��ѧС��Ϊ�о������������ʵ��ԭ��������֪2NO(g)+O2(g)
2NO2(g)������ȴ�����¶ȵ����߶���С��ij��ѧС��Ϊ�о������������ʵ��ԭ��������֪2NO(g)+O2(g) <0
<0 2NO2(g)���ʵ��Ƿ�Ӧ�ڣ���Ӧ�ٵĻ��E1�뷴Ӧ�ڵĻ��E2�Ĵ�С��ϵΪE1 E2(�>������<����=��)���������ʷ��̷����������¶ȸ÷�Ӧ���ʼ�С��ԭ���� ��
2NO2(g)���ʵ��Ƿ�Ӧ�ڣ���Ӧ�ٵĻ��E1�뷴Ӧ�ڵĻ��E2�Ĵ�С��ϵΪE1 E2(�>������<����=��)���������ʷ��̷����������¶ȸ÷�Ӧ���ʼ�С��ԭ���� ��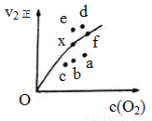
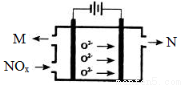
 NH3��H2O+H+
NH3��H2O+H+ H3O����CO32��
H3O����CO32�� 2H2O��CO2��
2H2O��CO2��