��Ŀ����
Ϊ�˳�ȥ������Ca2+��Mg2+��SO42-�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£������ڳ������Լ��Թ�����

��1���ж�BaCl2�ѹ����ķ�����
��2���ڢܲ��У���ص����ӳ�ʽ��
��3�������������PH�ٹ��ˣ����ʵ������Ӱ����

��1���ж�BaCl2�ѹ����ķ�����
ȡ������Һ���ϲ���Һ1��2���ڵζ����ϣ��ٵ���1��2��BaCl2��Һ������Һδ����ǣ�����BaCl2�ѹ���
ȡ������Һ���ϲ���Һ1��2���ڵζ����ϣ��ٵ���1��2��BaCl2��Һ������Һδ����ǣ�����BaCl2�ѹ���
����2���ڢܲ��У���ص����ӳ�ʽ��
Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3��
Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3��
����3�������������PH�ٹ��ˣ����ʵ������Ӱ����
��
��
����С����ޡ������Է���ԭ���ڴ���������£����в��ֳ����ܽ⣬�Ӷ�Ӱ���Ƶþ��εĴ���
�ڴ���������£����в��ֳ����ܽ⣬�Ӷ�Ӱ���Ƶþ��εĴ���
����������1���Ȼ�������ʱ����������������ɫ������
��2�����ε��ᴿ�У�����̼���Ƶ������dz�ȥ�������Ӹ������Լ������ı����ӣ�
��3��Mg��OH��2��CaCO3��BaCO3�������ᷴӦ�������Ȼ�þ���Ȼ��ơ��Ȼ��������ʣ���Ӱ���Ƶþ��εĴ��ȣ�
��2�����ε��ᴿ�У�����̼���Ƶ������dz�ȥ�������Ӹ������Լ������ı����ӣ�
��3��Mg��OH��2��CaCO3��BaCO3�������ᷴӦ�������Ȼ�þ���Ȼ��ơ��Ȼ��������ʣ���Ӱ���Ƶþ��εĴ��ȣ�
����⣺��1���ڴ����ᴿʱ������������Ȼ�����Ŀ���dz�ȥ��������ӣ�����֮�������Ȼ�������ʱ����������������ɫ����������ͨ�����鱵������ȷ���Ȼ����Ƿ����������ȡ������Һ���ϲ���Һ1��2���ڵζ����ϣ��ٵ���1��2��BaCl2��Һ������Һδ����ǣ�����BaCl2�ѹ�����
�ʴ�Ϊ��ȡ������Һ���ϲ���Һ1��2���ڵζ����ϣ��ٵ���1��2��BaCl2��Һ������Һδ����ǣ�����BaCl2�ѹ���
��2�����ε��ᴿ�У�����̼���Ƶ������dz�ȥ�������Ӹ������Լ������ı����ӣ���Ӧ�ķ���ʽΪ��CaCl2+Na2CO3=CaCO3��+2NaCl��BaCl2+Na2CO3=BaCO3��+2NaCl����Ӧ��ʵ���ǣ�Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3����
�ʴ�Ϊ��Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3����
��3�������������pH�ٹ��ˣ�����Mg��OH��2��CaCO3��BaCO3�������ᷴӦ������������ˮ���Ȼ�þ���Ȼ��ơ��Ȼ��������ʣ��Ӷ�Ӱ���Ȼ��ƵĴ��ȣ�
�ʴ�Ϊ���У��ڴ���������£����в��ֳ����ܽ⣬�Ӷ�Ӱ���Ƶþ��εĴ��ȣ�
�ʴ�Ϊ��ȡ������Һ���ϲ���Һ1��2���ڵζ����ϣ��ٵ���1��2��BaCl2��Һ������Һδ����ǣ�����BaCl2�ѹ���
��2�����ε��ᴿ�У�����̼���Ƶ������dz�ȥ�������Ӹ������Լ������ı����ӣ���Ӧ�ķ���ʽΪ��CaCl2+Na2CO3=CaCO3��+2NaCl��BaCl2+Na2CO3=BaCO3��+2NaCl����Ӧ��ʵ���ǣ�Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3����
�ʴ�Ϊ��Ca2++CO32-=CaCO3����Ba2++CO32-=BaCO3����
��3�������������pH�ٹ��ˣ�����Mg��OH��2��CaCO3��BaCO3�������ᷴӦ������������ˮ���Ȼ�þ���Ȼ��ơ��Ȼ��������ʣ��Ӷ�Ӱ���Ȼ��ƵĴ��ȣ�
�ʴ�Ϊ���У��ڴ���������£����в��ֳ����ܽ⣬�Ӷ�Ӱ���Ƶþ��εĴ��ȣ�
���������⿼�����йش��ε��ᴿ֪ʶ�����Ը�����ѧ֪ʶ���лش���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
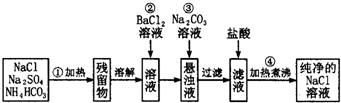
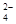 ����ɳ���ɽ���������ˮ��Ȼ������������������������ȷ�IJ���˳����
����ɳ���ɽ���������ˮ��Ȼ������������������������ȷ�IJ���˳����