��Ŀ����
(8��)�����Ǵ��ڹ����еĵ�λ���������Ǵӿ�ѧ��չ�ĽǶ��������趼��������Ҫ�����á�
��1������ʹ�ù����β�Ʒ(�մɵ�)����ʷ�Ѿ���һ�����ˣ�����1823��Ż�õ��ʹ裬��仯ѧ�ұ�������˹�ý����ػ�ԭSiF4��õ��ʹ裬д����ѧ����ʽ_______________ ��
��2��������ȡ�����������IJ�ͬ���õ��ĵ��ʹ���̬��ͬ��������Ҳ��ͬ��
�������ȷ���ԭ��������Ƶýϴ����ľ���裺4Al��3K2SiF6����3Si��2KAlF4��2K2AlF5�����ڸ÷�Ӧ��˵����ȷ����________(�����)��
���ð�ɰ����þ�ۻ���ڸ��������µõ����ι裬��Ӧ�Ļ�ѧ����ʽΪ___________ ��
��3����Ұ�⣬Ϊ��Ѹ�ٵõ��������ù��������Ca(OH)2��NaOH��ϣ�����ǿ�ȣ�����Ѹ�ٵõ�H2��Na2SiO3��CaO�����ֻ����������������д���÷�Ӧ�Ļ�ѧ����ʽ___________________________��
��1������ʹ�ù����β�Ʒ(�մɵ�)����ʷ�Ѿ���һ�����ˣ�����1823��Ż�õ��ʹ裬��仯ѧ�ұ�������˹�ý����ػ�ԭSiF4��õ��ʹ裬д����ѧ����ʽ_______________ ��
��2��������ȡ�����������IJ�ͬ���õ��ĵ��ʹ���̬��ͬ��������Ҳ��ͬ��
�������ȷ���ԭ��������Ƶýϴ����ľ���裺4Al��3K2SiF6����3Si��2KAlF4��2K2AlF5�����ڸ÷�Ӧ��˵����ȷ����________(�����)��
| A��Al�ǻ�ԭ�� | B����������ֻ��KAlF4 |
| C��ÿת��6NA���ӣ��õ�42 g Si | D��AlԪ����KAlF4��K2AlF5�л��ϼ۲�ͬ |
��3����Ұ�⣬Ϊ��Ѹ�ٵõ��������ù��������Ca(OH)2��NaOH��ϣ�����ǿ�ȣ�����Ѹ�ٵõ�H2��Na2SiO3��CaO�����ֻ����������������д���÷�Ӧ�Ļ�ѧ����ʽ___________________________��
��1��4K��SiF4===Si��4KF
��2����AC����2Mg��SiO2����Si��2MgO
��3�� Si��2NaOH��Ca(OH)2����Na2SiO3��CaO��2H2��
��2����AC����2Mg��SiO2����Si��2MgO
��3�� Si��2NaOH��Ca(OH)2����Na2SiO3��CaO��2H2��
�����������1�������ػ�ԭSiF4��õ��ʹ�ͬʱ����KF���ݴ�д����ʽ����2������������Ӧ��Al���ϼ����ߣ�����ԭ������������ΪKAlF4��K2AlF5��������������Al��Ϊ+3�ۣ�ÿ����3molSi��ת��12mol���ӣ���ת��6NA���ӣ��õ�42 g Si����3�����������Ca(OH)2��NaOH��ϣ���ǿ������H2��ͬʱ��Ӧ����CaO�����ơ�

��ϰ��ϵ�д�
�����Ŀ
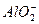 +2H2O
+2H2O ====CaCO3����
====CaCO3����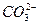 ��2H2O
��2H2O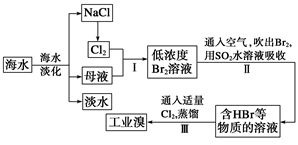
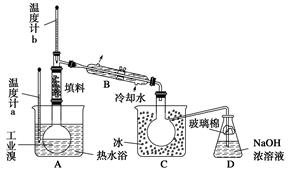
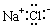
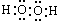
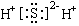

 CH3COO-+NH4+
CH3COO-+NH4+