��Ŀ����
��9�֣��л���A��B��C����ͼ��ʾת����ϵ��A�ķ���ʽΪC3H4O2��A����Br2�����Ȼ�̼��Һ�����ӳɷ�Ӧ��1 mol A����1molNaHCO3��Һǡ����ȫ��Ӧ��B����Ԫ��������A��ͬ����Է�������Ϊ46������̼����������Ϊ
52.2���������������Ϊ13�����Իش��������⣺

��1��A�����������ŵ�����Ϊ ��
��2��B�ķ���ʽΪ ��B��ͬϵ��D����Է�������Ϊ60����D���ܵĽṹ��ʽΪ ��
��3��A��B��Ӧ����C�Ļ�ѧ����ʽΪ ��
�÷�Ӧ���� ��Ӧ��
��4��A��B�Ļ���ﹲ1mol�����۶����Ժ��ֱ�����ϣ���ȫȼ��ʱ������ʼ��Ϊ��ֵ���� ��
a. ������������ b. ����ˮ���� c. ���ɶ�����̼����
52.2���������������Ϊ13�����Իش��������⣺

��1��A�����������ŵ�����Ϊ ��
��2��B�ķ���ʽΪ ��B��ͬϵ��D����Է�������Ϊ60����D���ܵĽṹ��ʽΪ ��
��3��A��B��Ӧ����C�Ļ�ѧ����ʽΪ ��
�÷�Ӧ���� ��Ӧ��
��4��A��B�Ļ���ﹲ1mol�����۶����Ժ��ֱ�����ϣ���ȫȼ��ʱ������ʼ��Ϊ��ֵ���� ��
a. ������������ b. ����ˮ���� c. ���ɶ�����̼����
��1��̼̼˫�����Ȼ���2�֣�
��2��
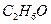 ��1�֣���
��1�֣���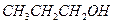 ��
��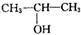 ��2�֣�
��2�֣���3��
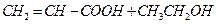
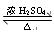
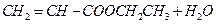
��������ȡ������3�֣�
��4��a��1�֣�
��

��ϰ��ϵ�д�
�����Ŀ
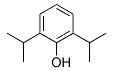
 ���Ҿ�Ϊ��״�ṹ������֪�л�����ͬһ��̼�����������ǻ����ȶ���
���Ҿ�Ϊ��״�ṹ������֪�л�����ͬһ��̼�����������ǻ����ȶ���
 _________________
_________________ �屽��ֲ���͢���ȩ������������Ӳ֬�������
�屽��ֲ���͢���ȩ������������Ӳ֬������� �Զ��ױ�
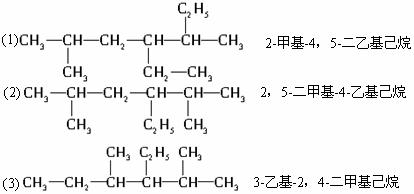
�Զ��ױ�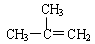 2������2����ϩ
2������2����ϩ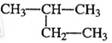 2���һ�����
2���һ�����