��Ŀ����
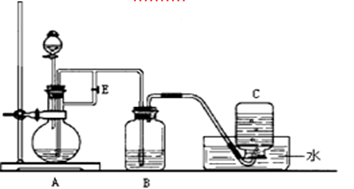
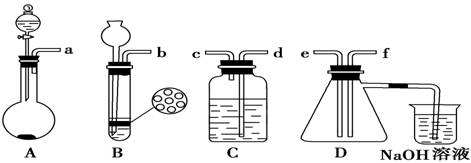
��ͼ��ʵ������ȡ��������ֳ�������װ�ã�
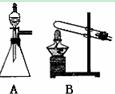
(1)ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼��Ӧѡ�õķ���װ
���� ����ѡ��A"��B"����
��Ӧ�����ӷ���ʽΪ
(2)������غͶ�����������ȡ������Ӧѡ�õķ���װ����________����ѡ��A"��B"����
��ѧ��Ӧ����ʽΪ����������������������������������������������
(3)����Aװ����ȡ��������ѡ�Լ�Ϊ ��
��Ӧ�Ļ�ѧ����ʽΪ
(4)��(2)(3)���ַ�����ȡ����ʱ����������ͬ��������������(2)(3)����Ӧת�Ƶĵ�����֮��Ϊ ��

(1)ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼��Ӧѡ�õķ���װ
���� ����ѡ��A"��B"����
��Ӧ�����ӷ���ʽΪ
(2)������غͶ�����������ȡ������Ӧѡ�õķ���װ����________����ѡ��A"��B"����
��ѧ��Ӧ����ʽΪ����������������������������������������������
(3)����Aװ����ȡ��������ѡ�Լ�Ϊ ��
��Ӧ�Ļ�ѧ����ʽΪ
(4)��(2)(3)���ַ�����ȡ����ʱ����������ͬ��������������(2)(3)����Ӧת�Ƶĵ�����֮��Ϊ ��
(1) A CaCO3 + 2H+ =Ca2++CO2��+2H2O
(2) B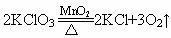
(3) H2O2��MnO2��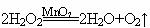
(4) 2:1
(2) B
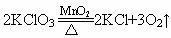
(3) H2O2��MnO2��
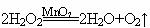
(4) 2:1
��

��ϰ��ϵ�д�
 ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�
�����Ŀ

 ��
��