��Ŀ����
��֪Ǧ���صĹ���ԭ��ΪPb+PbO2+2H2SO4
2PbSO4+2H2O���������ͼ1��ʾװ�ý��е�⣨���Һ����������õ�Ǧ������ת��0.4mol����ʱ���缫��������С11.2g����ش��������⣺
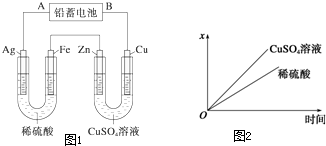
��1��A��Ǧ���ص�
��2��Ag�缫�ϵķ�ӦʽΪ
��3��Cu�缫�ϵķ�ӦʽΪ
��4����ͼ2��ʾ���ʱij������������x����ʱ��仯�����ߣ������x���п��ܱ�ʾ����
a������U����������������
b������U�ι������������ļ�����
c������U���������������������
| �ŵ� | ��� |

��1��A��Ǧ���ص�
��
��
�����������������Ǧ���ص�������ӦʽΪPbO2+4H++SO42-+2e-�TPbSO4+2H2O
PbO2+4H++SO42-+2e-�TPbSO4+2H2O
����2��Ag�缫�ϵķ�ӦʽΪ
2H++2e-�TH2��
2H++2e-�TH2��
�����������ʹ�0.4
0.4
g����3��Cu�缫�ϵķ�ӦʽΪ
Cu-2e-�TCu2+
Cu-2e-�TCu2+
��CuSO4��Һ��Ũ������
����
�����С�����������䡱������4����ͼ2��ʾ���ʱij������������x����ʱ��仯�����ߣ������x���п��ܱ�ʾ����
b
b
������ţ���a������U�ι����������������
b������U�ι������������ļ�����
c������U���������������������
��������1����Ǧ������ת��0.4mol����ʱ���缫��������С11.2g��˵��������������������������ԭ��ظ����ĵ缫������������ԭ��������ĵ缫��������ԭ��ظ�����ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ��
��2���������������ϡ����ʱ�������������ӷŵ���������������ת�Ƶ����غ���㣻
��3��ͭ��������������ͭʧ���ӷ���������Ӧ������������ͭ�����Ը�װ���ǵ�Ƴأ�
��4�����ʱ��a���ұ�U�ιܲ��������壻
b��ͭ��Ħ��������������
c�����U�ι������������ұ�U�ι�����ͭ��
��2���������������ϡ����ʱ�������������ӷŵ���������������ת�Ƶ����غ���㣻
��3��ͭ��������������ͭʧ���ӷ���������Ӧ������������ͭ�����Ը�װ���ǵ�Ƴأ�
��4�����ʱ��a���ұ�U�ιܲ��������壻
b��ͭ��Ħ��������������
c�����U�ι������������ұ�U�ι�����ͭ��
����⣺��1����Ǧ������ת��0.4mol����ʱ���缫��������С11.2g��˵������������������������������ԭ��ظ���������A�Ǹ�����B�������������϶�����Ǧ�õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪPbO2+4H++SO42-+2e-�TPbSO4+2H2O��
�ʴ�Ϊ������PbO2+4H++SO42-+2e-�TPbSO4+2H2O��
��2���������������ϡ����ʱ�������������ӷŵ������������缫��ӦʽΪ2H++2e-�TH2������������������=
��2g/mol=0.4mol��
�ʴ�Ϊ��2H++2e-�TH2����0.4��
��3��ͭ��������������ͭʧ���ӷ���������Ӧ���缫��ӦʽΪCu-2e-�TCu2+������������ͭ�����Ը�װ���ǵ�Ƴأ��������Һ��ͭ����Ũ�Ȳ��䣬
�ʴ�Ϊ��Cu-2e-�TCu2+�����䣻��
��4��a���ұ�U�ιܲ��������壬���U�ι��������壬����ϡ�����������������������ͭ��Һ���ʴ���
b����ת����ȵ���ʱ���ܽ���������ʵ�����ȣ�ͭ��Ħ�������������������ұ�U�ι��������ٵ������������U�ι��������ٵ�����������ȷ��
c����ת����ȵ���ʱ���������ʵ����ʵ�����ȣ���ͭ��Ħ�����������������������U�ι���������������С���ұ�U�ι�����ͭ���������ʴ���
�ʴ�Ϊ��b��
�ʴ�Ϊ������PbO2+4H++SO42-+2e-�TPbSO4+2H2O��
��2���������������ϡ����ʱ�������������ӷŵ������������缫��ӦʽΪ2H++2e-�TH2������������������=
| 0.4mol |
| 2 |
�ʴ�Ϊ��2H++2e-�TH2����0.4��
��3��ͭ��������������ͭʧ���ӷ���������Ӧ���缫��ӦʽΪCu-2e-�TCu2+������������ͭ�����Ը�װ���ǵ�Ƴأ��������Һ��ͭ����Ũ�Ȳ��䣬
�ʴ�Ϊ��Cu-2e-�TCu2+�����䣻��
��4��a���ұ�U�ιܲ��������壬���U�ι��������壬����ϡ�����������������������ͭ��Һ���ʴ���
b����ת����ȵ���ʱ���ܽ���������ʵ�����ȣ�ͭ��Ħ�������������������ұ�U�ι��������ٵ������������U�ι��������ٵ�����������ȷ��
c����ת����ȵ���ʱ���������ʵ����ʵ�����ȣ���ͭ��Ħ�����������������������U�ι���������������С���ұ�U�ι�����ͭ���������ʴ���
�ʴ�Ϊ��b��
���������⿼����ԭ��غ͵���ԭ������ȷ�ж�ԭ����������ǽⱾ��ؼ����������ӷŵ�˳��ȷ���������缫��Ӧ������ϵ�ʧ�����غ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 2PbSO4��2H2O
2PbSO4��2H2O 2PbSO4��2H2O
2PbSO4��2H2O