��Ŀ����
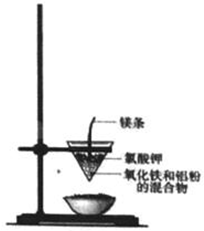
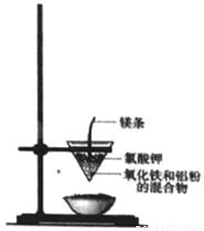
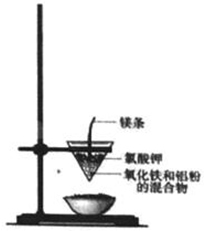 ���ֽ�����ұ������ͨ���ٸ����·�����������ԭ��Ӧ����ɵģ�ʵ�����������ȷ�Ӧ��ģ������ұ��������ʵ��װ����ͼ��
���ֽ�����ұ������ͨ���ٸ����·�����������ԭ��Ӧ����ɵģ�ʵ�����������ȷ�Ӧ��ģ������ұ��������ʵ��װ����ͼ����1�����ȷ�Ӧ�Ļ�ѧ����ʽΪ
Fe2O3+2Al
2Fe+Al2O3
| ||
Fe2O3+2Al
2Fe+Al2O3
��
| ||
��2�����������ȷ�Ӧ��������
��Ӧ�ų��������ȣ�������ҫ�۵Ĺ⣬ֽ©�����²����մ���������������ɳ��
��Ӧ�ų��������ȣ�������ҫ�۵Ĺ⣬ֽ©�����²����մ���������������ɳ��
����3���е�ͬѧ�Ʋ⣬���ȷ�Ӧ���õ����������������Ͻ����һ����ʵ�鷽����֤�����õ������к��н���������ʵ�����õ��Լ���
NaOH��Һ
NaOH��Һ
�����۲쵽����������
����������
����ʱ��˵���������к��н���������������1���������������ڸ��������·������ȷ�Ӧ����������������
��2����Ӧ�ڸ��������½��У����ų��������ȣ������������ɣ�
��3�����ݽ���������NaOH��Һ��Ӧ��������ѡ��ʵ�鷽����
��2����Ӧ�ڸ��������½��У����ų��������ȣ������������ɣ�
��3�����ݽ���������NaOH��Һ��Ӧ��������ѡ��ʵ�鷽����
����⣺��1���������������ڸ��������·������ȷ�Ӧ������������������Ӧ�Ļ�ѧ����ʽΪFe2O3+2Al
2Fe+Al2O3���ʴ�Ϊ��Fe2O3+2Al
2Fe+Al2O3��
��2����Ӧ�ڸ��������½��У����ų��������ȣ�þ��ȼ��ʱ����ҫ�۵İ⣬�����������ɣ�
�ʴ�Ϊ����Ӧ�ų��������ȣ�������ҫ�۵Ĺ⣬ֽ©�����²����մ���������������ɳ�У�
��3������������NaOH��Һ��Ӧ��������������֤�����õ������к��н�������ѡ��NaOH��Һ�������������ɣ���˵��Ϊ�����Ͻ𣬹ʴ�Ϊ��NaOH��Һ�����������ɣ�
| ||
| ||
��2����Ӧ�ڸ��������½��У����ų��������ȣ�þ��ȼ��ʱ����ҫ�۵İ⣬�����������ɣ�
�ʴ�Ϊ����Ӧ�ų��������ȣ�������ҫ�۵Ĺ⣬ֽ©�����²����մ���������������ɳ�У�
��3������������NaOH��Һ��Ӧ��������������֤�����õ������к��н�������ѡ��NaOH��Һ�������������ɣ���˵��Ϊ�����Ͻ𣬹ʴ�Ϊ��NaOH��Һ�����������ɣ�
���������⿼�����ȷ�Ӧ����Ŀ�ѶȲ���ע�����ȷ�Ӧ��ԭ����ʵ����������Լ���Ӧ����ѧϰ��ע����ػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ