��Ŀ����
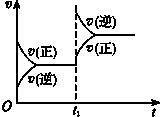
14����a��b����������Ҽ����ȵ��ܱ�������a�����ݻ����䣬b�еĻ����������ƶ����Ա�������ѹǿ��ȣ�����ͬ�����½�3mol A��1mol B�ֱ�ͬʱ�����a��b�������У�������Ӧ��3A��g��+B��g��?2C��g��+D��g����1���ﵽƽ��ʱ��a��A��Ũ��ΪM mol/L��A��ת����ΪN��b��A��Ũ��Ϊm mol/L��A��ת����Ϊn����M��m��N��n���������=�����Ƚϣ�
��2�������¶Ȳ��䣬��������ȷֱ����a��b����������ƽ���a��A��Ũ��ΪM mol•L-1����D��E
A 6mol A+2mol B B 3mol A+2mol C C 2mol C+1mol B+1mol D
D 2mol C+1mol D E 1.5mol C+0.5mol B+1mol C+0.5mol D��
���� ��1��a�����ݻ����䣬Ϊ���ݱ仯��b��������ѹǿ��ȣ�Ϊ��ѹ�仯��������Ӧ3A��g��+B��g��?2C��g��+D��g�����������ʵ������ٵķ�Ӧ����b�൱����aƽ��Ļ����ϼ�ѹ��ƽ�����ƣ�ƽ���ƶ���������A��Ũ����������ƽ��ʱ��Ũ�ȸ���ԭƽ�⣬�ݴ˽��
��2��Ҫ��ﵽƽ���a��A��Ũ��ΪM mol•L-1����ԭ��ϵ�ǵ�Чƽ�⣬���µ��ݵ������£���ͬ���ʵ�Ͷ������ȣ�
��� �⣺��1��a�����ݻ����䣬Ϊ���ݱ仯��b��������ѹǿ��ȣ�Ϊ��ѹ�仯��������Ӧ3A��g��+B��g��?2C��g��+D��g�����������ʵ������ٵķ�Ӧ����b�൱����aƽ��Ļ����ϼ�ѹ��ƽ�����ƣ�����b��A��ת���ʴ�N��n��ƽ���ƶ���������A��Ũ����������ƽ��ʱ��Ũ�ȸ���ԭƽ�⣬��b��ƽ��ʱA��Ũ�ȴ���a��A��ƽ��Ũ�ȣ���M��m���ʴ�Ϊ����������
��2�������¶Ȳ��䣬�����б����ֱ����a��b�������У��ﵽƽ���a��C��Ũ����ΪN mol•L-1��һ�ߵ�ת��Ϊͬһ���ʣ����ʵ�����ͬD��2molC+1molD E��1.5molA+0.5molB+1molC+0.5molD�൱��3mol A��1mol B����ѡD��E��
���� ���⿼�黯ѧƽ���ƶ�����㣬�Ѷ��еȣ�ע�����õ�Ч˼�����ƽ�⽨����;������1����A��Ũ�ȱȽ�Ϊ�״��㣬ѧ��������ƽ���ƶ������ж�a��Ũ�ȴ�������ļ�С��ע�����ƽ���ƶ�ԭ����ƽ�ⳣ�����⣮

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�| A�� | 0.04 mol/L | B�� | 0.2 mol/L | C�� | 1 mol/L | D�� | 2 mol/L |
| A�� | ������ | B�� | �Զ������� | C�� | ��������� | D�� | �������� |
| A�� | ������ѹǿ������ʱ����ı�ʱ | |
| B�� | ��������������ٸı�ʱ | |
| C�� | ��������и���ɳɷֵĺ������ٸı�ʱ | |
| D�� | ��λʱ����ÿ����1 mol I2��ͬʱ��2 mol HI����ʱ |
| A�� | ��A��ʾ�ķ�Ӧ������0.4mol/��L•min�� | |
| B�� | �ֱ���B��C��D��ʾ�ķ�Ӧ�������ֵ��3��2��1 | |
| C�� | 2minĩʱ�ķ�Ӧ���ʣ���B��ʾ��0.3mol/��L•min�� | |
| D�� | ��B=0.3mol/��L•min���ͦ�C=0.2mol/��L•min��������ʾ�÷�Ӧ����һʱ���ڵķ�Ӧ���� |
| A�� | F2��Cl2��Br2��I2���۵㡢�е������� | |
| B�� | HF��HCl��HBr��HI�����ȶ������μ��� | |
| C�� | Na��Mg��Al�Ļ�ԭ�����μ��� | |
| D�� | NaF��NaCl��NaBr��NaI���۵����ν��� |
| A�� | ʯī�����ʯ�;���趼����ԭ�Ӿ��� | |
| B�� | ���ʯ����ʯī�ȶ� | |
| C�� | �������Ľ��ʯ��ʯī��ȫȼ�գ��ų�������һ���� | |
| D�� | �������Ľ��ʯ��ʯī��ȫȼ�գ�ʯī�ų��������� |
| A�� | A��B�������壬C��D���������� | B�� | A��B�������壬C��D��һ�������� | ||
| C�� | C��D�������壬A��B�������� | D�� | C��D�������壬A��B��һ�������� |