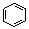
ћвƒњƒЏ»Ё
ѕ¬Ѕ– µ—йƒ№їсµ√≥…є¶µƒ «
| A£Ѓѕт““Ћб““х•÷–Љ”»л„гЅњµƒ30% NaOH»№“Ї£ђ‘ЏЉ”»»µƒћхЉюѕ¬єџ≤мх•≤гµƒѕы І |
| B£Ѓ““іЉ”л≈®ЅтЋбїмЇѕ£ђЉ”»»÷Ѕ140°ж÷∆»°““ѕ© |
| C£Ѓѕт2 mLµƒЉ„±љ÷–Љ”»л3µќKMnO4Ћб–‘»№“Ї£ђ”√Ѕ¶’сµі£ђєџ≤м»№“Ї—’…ЂЌ »• |
| D£Ѓ1 mol°§L£≠1 CuSO4»№“Ї2 mLЇЌ0.5 mol°§L£≠1 NaOH»№“Ї2mLїмЇѕЇуЉ”»л40%µƒ““»©»№“Ї0.5mL£ђЉ”»»÷уЈ–єџ≤м„©Їм…Ђ≥Ѕµнµƒ…ъ≥… |
AC
‘ћвЈ÷ќц£Ї‘Џ«в—хїѓƒ∆»№“Ї÷–““Ћб““х•µƒЋЃљвЈі”¶ƒ№Ќк»Ђљш––µљµ„£ђA’э»Ј£їB≤ї’э»Ј£ђ”¶Є√ «Љ”»»µљ170°ж£їЉ„±љƒ№ єЋб–‘Єя√ћЋбЉЎ»№“ЇЌ …Ђ£ђC’э»Ј£їD÷–«в—хїѓƒ∆ «≤ї„гµƒ£ђЇЌ““»©Јі”¶µ√≤їµљЇм…Ђ≥Ѕµн£ђD≤ї’э»Ј£ђір∞Є—°AC°£
µг∆ј£ЇЄ√ћв «ЄяњЉ÷–µƒ≥£Љыћв–Ќ£ђ ф”Џ÷–µ»ƒ—ґ»µƒ ‘ћв°£ ‘ћвƒ—“„ ÷–£ђїщі°–‘«њ°£”–јы”Џ≈а—ш—І…ъєжЈґ—ѕљчµƒ µ—й…иЉ∆ƒ№Ѕ¶£ђ“≤”–÷ъ”Џµчґѓ—І…ъµƒ—Іѕ∞–Ћ»§ЇЌ—Іѕ∞їэЉЂ–‘£ђ”–јы”Џћб…э—І…ъµƒ—Іњ∆ЋЎ—ш°£

ЅЈѕ∞≤бѕµЅ–ір∞Є
 ‘ƒґЅњм≥µѕµЅ–ір∞Є
‘ƒґЅњм≥µѕµЅ–ір∞Є
ѕаєЎћвƒњ