��Ŀ����
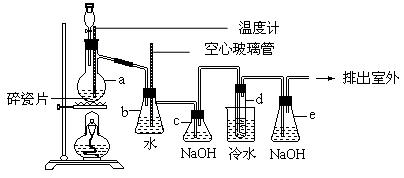
��12�֣�1,2������������������������Ӽ���������Ϊ��ɫҺ�壬�ܶ�Ϊ2.18g cm��3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѣ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾ�豸�Ʊ�1,2���������顣ͼ�з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ�壨���渲������ˮ�����ݴ���ش��������⣺
cm��3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѣ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾ�豸�Ʊ�1,2���������顣ͼ�з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ�壨���渲������ˮ�����ݴ���ش��������⣺
��1��д���Ʊ�1,2�����������������Ӧ����ʽ��
______________________________________�� ��
��2����ȫƿb���Է�ֹ�����������ڼ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱb������_________________________________________��
��3������c��NaOH��Һ�������ǣ� __________________________________��
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³����ܶࡣ���װ�õ�������û�����⣬�Է�������ܵ�ԭ�� ��
��5��eװ����NaOH��Һ�������ǣ�__________________________________��
 cm��3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѣ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾ�豸�Ʊ�1,2���������顣ͼ�з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ�壨���渲������ˮ�����ݴ���ش��������⣺
cm��3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѣ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾ�豸�Ʊ�1,2���������顣ͼ�з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ�壨���渲������ˮ�����ݴ���ش��������⣺
��1��д���Ʊ�1,2�����������������Ӧ����ʽ��
______________________________________�� ��
��2����ȫƿb���Է�ֹ�����������ڼ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱb������_________________________________________��
��3������c��NaOH��Һ�������ǣ� __________________________________��
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³����ܶࡣ���װ�õ�������û�����⣬�Է�������ܵ�ԭ�� ��
��5��eװ����NaOH��Һ�������ǣ�__________________________________��
��1��CH3CH2OH 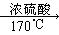 CH2==CH2�� + H2O
CH2==CH2�� + H2O
CH2==CH2 + Br2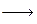 CH2BrCH2Br
CH2BrCH2Br
��2��bƿ��ˮ���½���������ˮ���������������
��3����ȥ���ܲ�������������
��4����ϩ��������̫�죻��Ӧ�¶�δѸ�ٴﵽ170��
��5�����ջӷ������壬��ֹ��Ⱦ
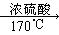 CH2==CH2�� + H2O
CH2==CH2�� + H2O CH2==CH2 + Br2
 CH2BrCH2Br
CH2BrCH2Br��2��bƿ��ˮ���½���������ˮ���������������
��3����ȥ���ܲ�������������
��4����ϩ��������̫�죻��Ӧ�¶�δѸ�ٴﵽ170��
��5�����ջӷ������壬��ֹ��Ⱦ
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 )����b��һ�ȴ����a��b����ֵ�ֱ�Ϊ
)����b��һ�ȴ����a��b����ֵ�ֱ�Ϊ 